May 8, 2008 (CIDRAP News) An antiviral drug being developed to provide protection against smallpox and related viruses performed well in the first test of its safety and activity in humans, according to a report in the May issue of Antimicrobial Agents and Chemotherapy.
The drug, called ST-246, was generally well tolerated and caused no serious adverse events in a study involving 38 volunteers, the report says. The doses used in the study produced plasma concentrations comparable to those that protected animals from infections of lethal orthopoxvirus (the family that includes smallpox) in preclinical experiments.
The study was conducted by scientists from Siga Technolgoies Inc., Corvallis, Ore., developer of ST-246, and two other private companies, with Siga's Robert Jordan as the lead author.
Currently, vaccination within the first 4 days after exposure is considered the only reliable treatment for smallpox, although the antiviral drug cidofovir has shown activity against orthopoxviruses. Smallpox disease was eradicated in the 1970s, but there is concern that secret stocks of the virus may still exist and fall into the hands of terrorists or rogue nations. (The only known stocks of the virus, kept for research, are held in government laboratories in the United States and Russia.)
ST-246 was identified in a screen of more than 350,000 compounds designed to find substances that could inhibit vaccinia virus, which is a close relative of smallpox virus and is used in smallpox vaccine. In preclinical studies, ST-246 protected mice from lethal infection with vaccinia virus, cowpox virus, and ectromelia virus, according to the new report. Also, in unpublished studies, the drug was active against monkeypox in squirrels and against both monkeypox and smallpox viruses in monkeys, the report says.
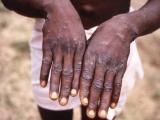
ST-246 was used on an emergency basis last year in a 2-year-old boy who became critically ill with eczema vaccinatum, a form of vaccinia virus infection. The boy, who survived, was infected through exposure to his father, a soldier who had received a smallpox shot.
In the safety trial, the researchers recruited 40 healthy adult volunteers and divided them into four groups. In three of the groups, volunteers who were fasting received ST-246 in a single oral dose of 500, 1,000, or 2,000 mg, or a placebo. Volunteers in the fourth group received either 1,000 mg of ST-246 or a placebo after consuming a high-fat meal. In all, 31 volunteers received the drug and 7 received a placebo.
Six of the 38 volunteers had an adverse event, such as neutropenia, constipation, back pain, or headache, but the incidence was higher in the placebo subjects (2 of 7, or 29%) than in the treated subjects (4 of 31, 13%). Some of the fasting volunteers had slight, transient decreases in white blood cell counts and neutrophil percentages 1 day after treatment, but this was seen in both the ST-246 and placebo groups.
Measurable plasma concentrations of the drug were found in all the treated volunteers from a half-hour to as long as 72 hours after administration, the report says. It took an average of 2 to 3 hours after dosing for the drug to reach maximum concentration. Food seemed to increase the plasma presence of the drug, as the nonfasting volunteers had 1.6-fold higher maximum concentration than the fasting volunteers.
Given the plasma concentrations observed in this study, the authors predict that ST-246 doses ranging from 400 to 800 mg will provide nonfasting humans with protection comparable to that seen in a study in nonhuman primates that received a high dose of monkeypox virus. In the animal subjects, an ST-246 dose of 10 mg/kg provided full protection from death.
"Administration of ST-246 resulted in exposure levels predicted to be sufficient for inhibiting orthopoxvirus replication compared to exposure levels in nonhuman primates in which ST-246 protected animals from lethal orthopoxvirus nfection," the report states.
Jordan R, Tien D, Bolken TC, et al. Single-dose safety and pharmacokinetics of ST-246, a novel orthopoxvirus egress inhibitor. Antimicrobial Agents Chemo 2008 May;52(5):1721-7 [Abstract]
See also:
May 17, 2007, CIDRAP News story "CDC reports on soldier's son who had severe vaccinia infection"
Mar 19, 2007, CIDRAP News story "Son of vaccinated soldier has severe vaccinia infection"