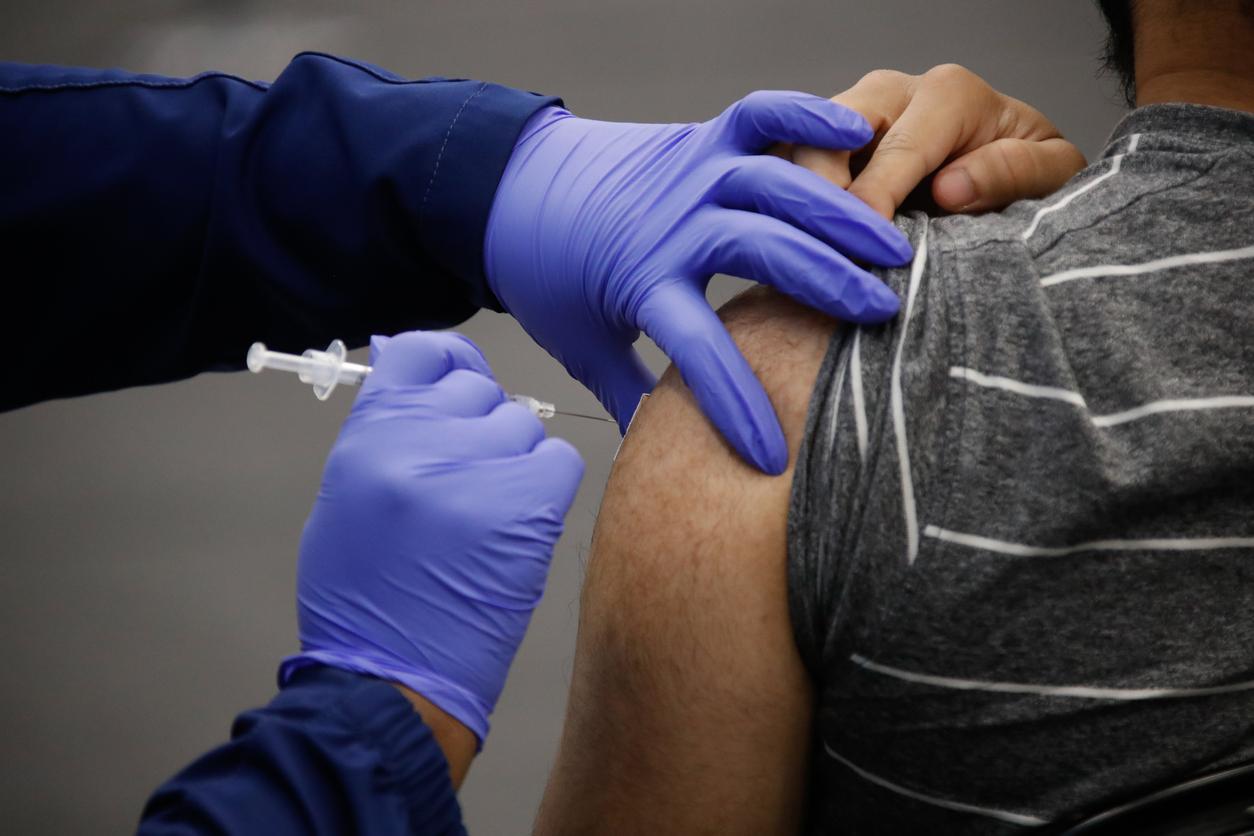
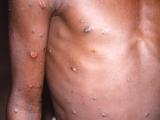
Moving beyond the use of the Jyennos mpox vaccine in outbreak settings, the vaccine advisory group to the Centers for Disease Control and Prevention (CDC) today unanimously recommended broader and more routine use to prevent the disease in people known to be at risk from the disease.
In February, the Advisory Committee for Immunization Practices (ACIP) voted in favor of using the two-dose vaccine series in outbreak settings. Though the public health emergency for mpox expired in the United States at the end of January, cases continue at a low but persistent level. Also, today’s decision helps set the stage for the eventual transition of mpox vaccine delivery, currently covered by the US government, to the commercial market.
Community spread persists, but vaccine uptake slows
During today’s ACIP meeting, Faisal Minhaj, PharmD, MPH, with the CDC's National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), said the disease has not been eliminated from the United States, and that from one to four cases are reported each day, with illnesses occurring with a broad geographic distribution. He said officials are seeing unlinked cases across regions, suggesting that the virus continues to spread in the community, along with the possibility that mpox is underdiagnosed.
Rare cases following vaccination, along with instances of reinfection, continue to be reported, but they make up a small proportion of reported cases, he said, adding that patients with such infections typically have few lesions and milder infections.
Vaccine uptake has slowed, and officials said uptake in risk groups is well below the level needed to keep population immunity from falling. So far, 1.25 million Jynneos doses have been administered, with 38.8% of the risk group with one-dose coverage and 24.3% having received two doses.
Recommendation details risk groups
The recommendation ACIP passed today is for people ages 18 and older who are at risk for mpox to receive the two-dose Jynneos series.
Risk groups include gay, bisexual, and other men who have sex with men and transgender or nonbinary people who in the past 6 months meet one or more of four criteria:
- A new diagnosis of one or more sexually transmitted disease
- More than one sex partner
- Sex at a commercial sex venue
- Sex linked to a large public event in a geographic region where mpox transmission is occurring
The recommendation also covers sexual partners of people in the risk groups and people who anticipate experiencing any situations that would raise their risk of contracting the virus.
During today’s voting, ACIP unanimously approved adding mpox vaccine for use in 18-year-olds to the Vaccines for Children program, a federally funded program that provides free vaccine to children who don't have health insurance or can't afford the cost. A National Institutes of Health trial is under way on the use of Jynneos in children ages 12 to 17, with results expected possibly in 2024.
ACIP’s recommendation now moves to the CDC director for final approval.
Anticipating transition to commercial sector
David Boucher, PhD, director of infectious disease preparedness and response at the Department of Health and Human Services (HHS) told the group that as the country moves past the emergency phase of mpox spread, the department supports a seamless transition to the commercial market.
In a statement today, Bavarian Nordic—maker of Jynneos—said pending final CDC approval of ACIP’s recommendations, it anticipated the commercial launch of the vaccine in the first half of 2024.
Paul Chaplin, PhD, Bavarian Nordic’s president and chief executive officer, said in the statement that ACIP’s broadened recommendation recognizes the importance of maintaining high awareness of the disease among risk groups and the importance of broader access beyond outbreak situations.
At today’s meeting, a Bavarian Nordic official said the wholesale acquisition cost per dose would likely be in the $200 to $270 range, with the price likely 25% to 30% less than that after contracting and reimbursement agreements are made.