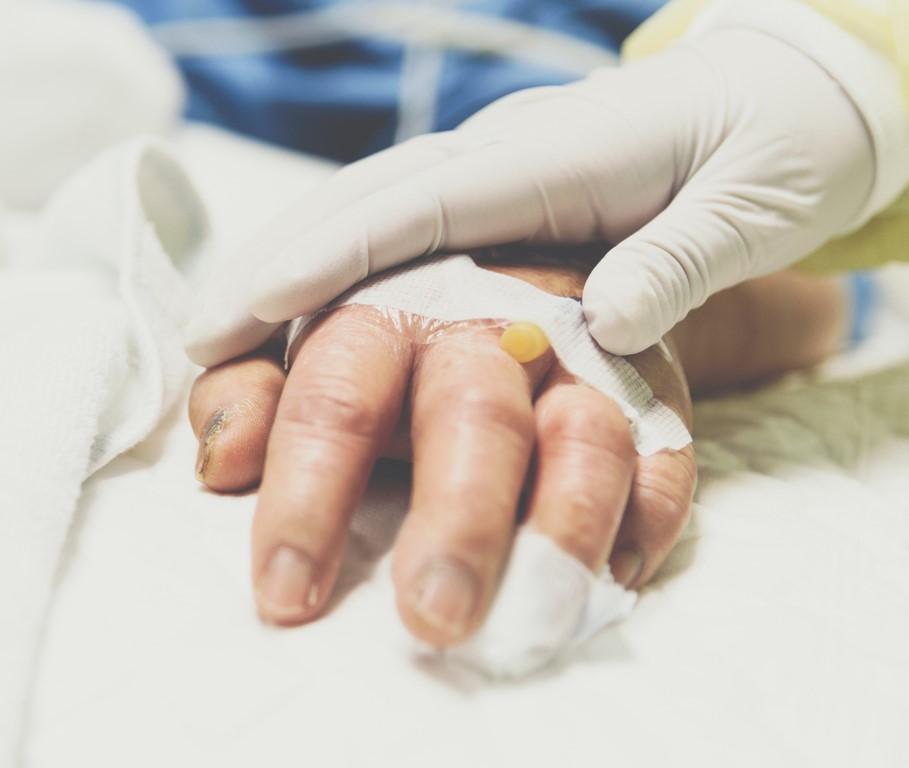
Convalescent plasma derived from recovered patients provided no benefit over usual care in 464 COVID-19 adult patients in India, according to the results of the first randomized, controlled trial to test the safety and effectiveness of the therapy.
The open-label, phase 2 PLACID trial, published yesterday in BMJ, involved assigning moderately ill coronavirus patients in 39 hospitals across India to receive either two doses of convalescent plasma 24 hours apart or standard care from Apr 22 to Jul 14.
Convalescent plasma is the liquid part of the blood taken from volunteers recovered from an infectious disease such as COVID-19 and transfused into sick patients in hopes that neutralizing antibodies in the plasma will help them recover. In this study, plasma antibody levels were measured at the end of the study rather than before.
Similar rates of death
Forty-four (19%) of the 235 patients assigned to receive convalescent plasma clinically deteriorated or died by 28 days, as did 41 (18%) of the 229 assigned to usual care (risk difference, 0.008).
Thirty-four of 228 patients (15%) who completed treatment in the convalescent plasma group and 31 of 226 patients (14%) who completed treatment in the usual-care group died. The deaths of 3 participants (1%) were recorded as possibly related to convalescent plasma.
Levels of neutralizing antibodies were available for 224 of 235 patients who received convalescent plasma; 160 (71.4%) received at least one unit of plasma with detectable antibody concentrations. There was no difference in clinical worsening or death in patients who received plasma with high or undetectable antibody concentrations or who received only usual care.
Patients receiving the therapy were more likely to experience relief from shortness of breath and fatigue and test negative for coronavirus after 7 days but did not show lower levels of inflammatory markers such as C-reactive protein, D-dimer, ferritin, or lactate dehydrogenase.
The authors called for future research to include antibody assays before the trial rather than only after to further clarify convalescent plasma's role in the treatment of coronavirus. "Convalescent plasma was not associated with a reduction in progression to severe COVID-19 or all cause mortality," they wrote. "This trial has high generalisability and approximates convalescent plasma use in real life settings with limited laboratory capacity."
Potential for blood clots
In a commentary in the same journal, Elizabeth Pathak, PhD, MSPH, of the Women's Institute for Independent Social Enquiry in Maryland, said that although convalescent plasma appeared to have relieved shortness of breath and fatigue faster than usual care, patients may have been influenced by knowing that they had received the therapy.
And although patients given convalescent plasma tested negative for COVID-19 at a 20% higher rate than usual-care patients on day 7, they showed no clinical benefit from the treatment, she said. Indeed, she added, convalescent plasma is not without risk and can lead to blood clots, which are also a complication of coronavirus.
Pathak said these risks should be thoroughly investigated and considered when designing future clinical trials, that only plasma with detectable levels of antibodies should be given to participants to ensure that all patients can receive any possible benefit, that future trials should be double-blinded with sham procedure controls, and that non-immune plasma shouldn't be used for controls because of its potential harms and the availability of lower-risk interventions such as normal saline.
"Finally, when multiple research teams require participants, triage committees are needed to direct recruitment away from low priority, duplicative, or underpowered trials with little potential for usable findings," Pathak wrote. "Institutions must guarantee that patients with COVID-19 are informed of all available trial options and assured autonomy in their decisions about participation."