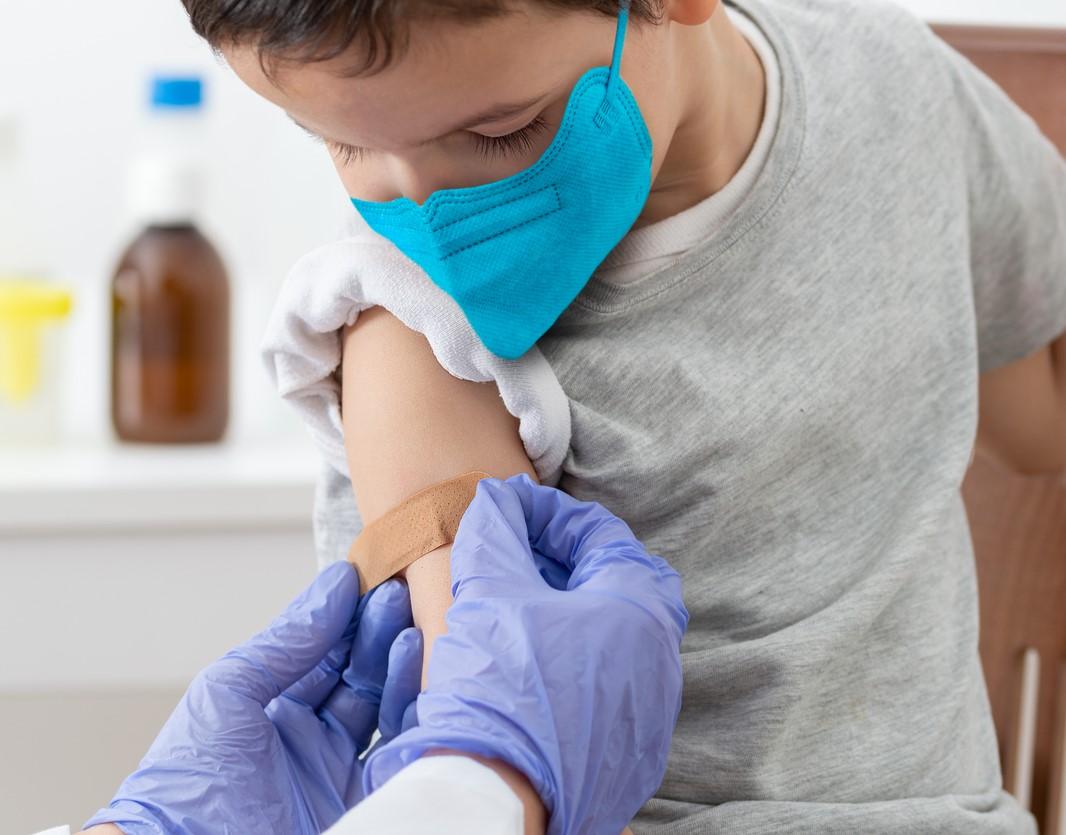
The Food and Drug Administration (FDA) today gave the thumbs-up to the emergency use of Pfizer COVID-19 boosters in kids ages 5 through 11, as the country's cases and hospitalizations show more signs of rises.
At the global level, the World Health Organization (WHO) today weighed in on booster doses of mRNA vaccine against as cases rise in four global regions.
CDC to consider FDA booster recommendation
In amending its earlier emergency use authorization (EUA) of the Pfizer-BioNTech vaccine, the FDA today authorized the use of a single booster in children ages 5 through 11 at least 5 months after completing the primary two-dose series.
In a statement, FDA Commissioner Robert Califf, MD, said though COVID-19 is typically less severe in children than adults, the Omicron surge came with higher numbers of illnesses, hospitalizations, and long-term effects from the disease in youngsters. He added that vaccination is still a strong hedge against severe consequences, and he urged parents to ensure that kids get their primary series.
Also, Peter Marks, MD, PhD, who directs the FDA's Center for Biologics Evaluation and Research, said emerging data show vaccine effectiveness wanes after the second dose in all authorized populations and that the benefits of a booster dose in younger kids outweighs potential risks. "A booster dose can help provide continued protection against COVID-19 in this and older age groups," he said.
Before the booster doses roll out in the 5- to 11-year-old age-group, vaccine advisors for the Centers for Disease Control and Prevention (CDC) need to make a formal recommendation. The Advisory Committee on Immunization Practices (ACIP) is slated to meet on May 19, presumably to consider the booster dose recommendation.
Rising cases in kids tracks with national activity
Last week, 93,000 more COVID-19 cases in children were reported, up 76% compared to 2 weeks ago, the American Academy of Pediatrics (AAP) said in its latest update. It added that cases in children have been on the rise for 5 weeks in a row.
The 7-day average for daily new cases has increased to 95,813, according to a New York Times analysis, part of a slow but steady rise that has been under way since the middle of April.
A more transmissible Omicron subvariant—BA.2.12.1—continues to make up a growing proportion of cases. In an update today, the CDC said that, nationally, BA.2.12.1 now makes up 47.5% of sequenced cases, up from 38.8% the previous week. The subvariant is now dominant in 4 of 10 Department of Health and Human Services (HHS) regions, mainly the Eastern Seaboard states and the central part of the Midwest that includes Iowa, Kansas, Missouri, and Nebraska.
Meanwhile, hospitalizations continue to rise and are up 26% over the past 2 weeks, according to the Times.
In related developments, amid high community transmission rising hospitalizations, New York City officials yesterday strongly recommended wearing masks in public indoor settings. Similarly, New Orleans health officials today strongly recommended wearing masks indoors because of rising COVID cases.
Owing to rising cases and the spread of a more transmissible subvariant, the Biden administration yesterday opened up a third round of free rapid COVID-19 test kits, with eight tests available for each household delivered by mail. In other federal developments, the FDA declined to authorize the antidepressant medication fluvoxamine as a treatment for COVID-19.
WHO: Second boosters show benefits for risk groups
In an interim statement today, the WHO's Strategic Advisory Group of Experts (SAGE) on Immunization said a review of data from seven studies suggests short-term benefits of an extra booster dose in some high-risk groups, such as healthcare workers, those older than 60, and those with weakened immune systems.
The experts emphasized that the data are only available for mRNA vaccines and that there isn't much data on duration of protection and benefits for younger age-groups. SAGE members urged countries that are considering second booster doses to balance financial considerations with the expected incremental benefits.
At media briefing today, WHO Director-General Tedros Adhanom Ghebreyesus, PhD, said that, over the past week, COVID-19 cases have risen in four of the WHO regions. Cases have been rising in the Africa and the Americas over the past few weeks, but the WHO dashboard now reflects small rises in the Western Pacific and Eastern Mediterranean regions.
In other global developments:
- The United Nations Human Rights Office today said it was deeply concerned about the human rights impact of North Korea's first officially reported COVID-19 outbreak and lockdown. It said spread of the virus in the absence of vaccination may have a devastating impact, as the country struggles with a limited health infrastructure, few tools for fighting the virus, and limited ability to provide people with basic needs, such as food.
- Shanghai has achieved its goal of going 3 days with no new COVID-19 cases outside of quarantine areas, according to Reuters. City officials recently announced a June phased easing of the 6-week lockdown.