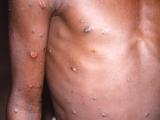
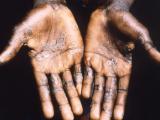
Today officials from the Department of Health and Human Services (HHS) and the Food and Drug Administration (FDA) declared the ongoing monkeypox outbreak in the United States a public health emergency, paving the way for an increase in funding for tests, vaccines, and treatments for the poxvirus.
HHS Secretary Xavier Becerra said the move would help take the federal response to the virus to the next level
"I ask each American to take monkeypox seriously," said Becerra.
The announcement of a public health emergency follows steps by some states and cities to make such declarations. The World Health Organization (WHO) declared monkeypox a public health emergency of international concern (PHEIC) at the end of July.
FDA to consider alternative dosing
FDA Commissioner Robert Califf, MD, said the FDA was considering authorizing fractional dosing of Bavarian Nordic's Jynneos, the only vaccine approved for use against monkeypox in the United States.
Intradermal injection at one-fifth the dose strength, as opposed to intramuscular injection, would stretch the supply of vaccine, which has been in high demand in virus hot spots such as New York City, Chicago, and San Francisco.
"This could be a promising approach," Califf said. "The goal has always been to vaccinate as many people as possible."
HHS has previously said the federal government has ordered 6.9 million doses of Jynneos, but the bulk of the vaccines will not be available until 2023.
More than 99% of the cases in the US are in men who have sex with men (MSM), and the Biden administration has been routinely criticized by gay rights and advocacy groups in recent weeks for doing too little to combat the spread of the virus.
Yesterday the Centers for Disease Control and Prevention (CDC) reported 291 more monkeypox cases, putting the nation's total at 6,617 cases in 50 jurisdictions.
Call for Tpoxx trials
In other news, an editorial in the New England Journal of Medicine today asks officials to consider clinical trials of tecovirimat (Tpoxx)—an antiviral drug approved for treating smallpox—to test for efficacy against monkeypox.
Scientists from the FDA, CDC, and National Institute of Allergy and Infectious Diseases asked for the drug to be tested under the FDA's Animal Rule.
"This pathway allows for approval of drugs for serious or life-threatening conditions when it is not ethical to conduct efficacy studies in humans and not feasible to conduct field trials to study the effectiveness of a drug or biologic product," they wrote. "Under the Animal Rule, efficacy is established on the basis of adequate and well-controlled studies in animal models of the human disease or condition of interest; safety must be adequately evaluated in people."
Drawing comparisons to the antiretrovirals used to fight HIV, the authors said these trials will provide data needed for clinical and regulatory decision making in the United States.