Feb 9, 2012
USDA will postpone ban on non-O157 E coli in beef until March
The US Department of Agriculture (USDA) will postpone its ban on six pathogenic non-O157 strains of Escherichia coli in certain beef products from March until Jun 4, Bloomberg News and other news services have reported. After studying the issue for 4 years, the USDA had announced plans last September to begin testing beef trim, used to make ground beef, in March. The meat industry has opposed the plan, arguing that the need is unclear and that tests for the strains are not ready. The Bloomberg story said the USDA postponed the step to give meat processors more time to make sure their own testing methods work. The agency is targeting E coli O26, O45, O103, O111, O121, and O145. The Centers for Disease Control and Prevention (CDC) has estimated that non-O157 E coli strains cause 112,000 illnesses per year, with the six targeted ones accounting for most of them, USDA officials said when they announced the
ban in September.
Feb 8 Bloomberg News story
Dec 1, 2011, CIDRAP News story
Sep 13, 2011, CIDRAP News story
Review of botulism outbreaks finds gaps in detection
A study of botulism outbreaks found that the gap between exposure and recognition varied widely, and that identification of links between sick patients plays a key role in detection. The findings were published yesterday in Foodborne Pathogens and Diseases by University of Minnesota researchers. Their study used CDC data on botulism illnesses and outbreaks that occurred from 1993 to 2008, along with outbreak reports published in medical journals. Of 416 botulism illnesses they identified, 204 (49%) were linked to outbreaks. Family connections and cohospitalizations were most common in large outbreaks, those involving more than 5 cases. The delay between exposure and recognition ranged from 48 to 216 hours. The researchers found that clustering patterns varied by food distribution type. Linkages between cases were more likely to be recognized when the contaminated food was locally or regionally distributed. They warned that
gaps in detection would delay confirmation of an intentionally contaminated product distributed widely and recommended that medical organizations and the Department of Homeland Security push for better training in detection.
Feb 8 Foodborne Pathog Dis abstract
Experts warn about untreatable gonorrhea
Three experts warned in the New England Journal of Medicine today that an untreatable type of gonococcal infection could be emerging, based on patterns they've noticed in drug resistance and epidemiology. The CDC's Gail Bolan, MD, University of North Carolina's P. Frederick Sparling, MD, and the University of Washington's Judith Wasserheit, MD, MPH, said the number of isolates that show resistance to cephalosporins has increased by a factor of 17, from 0.1% in 2006 to 1.7% in the first half of 2011. Resistance has been particularly noteworthy in cases from the western United States and in men who have sex with men. They noted that the pattern is worrisome, because it resembles those seen during the emergence of fluoroquinolone-resistant Neisseria gonorrhoeae. They wrote that it's not known if higher doses of cephalosporins can curb the threat of resistant strains, and they called for the nation to rebuild its defenses against the
disease.
Feb 9 N Engl J Med extract
FDA warns of C difficile risk associated with stomach-acid drugs
The US Food and Drug Administration (FDA) issued an alert yesterday that proton-pump inhibitors (PPIs), which are drugs to reduce stomach acid, may be associated with an increased risk of Clostridium difficile–associated diarrhea (CDAD). It said, "A diagnosis of CDAD should be considered for patients taking PPIs who develop diarrhea that does not improve." The FDA said in the alert that it is working with manufacturers to include information about the increased risk on PPI labels. The agency reached its conclusion after reviewing reports from its Adverse Event Reporting System and from 28 observational studies, 23 of which showed the increased risk associated with PPIs. The FDA also said in the statement that it is exploring a possible association between CDAD and histamine H2 receptor blockers, which are used to treat gastroesophageal reflux, ulcers, and heartburn.
Feb 8 FDA announcement
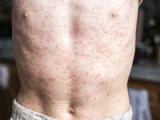
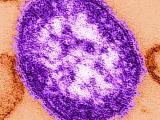
Indiana reports 2 confirmed, 2 probable measles cases, Super Bowl link
Two people in Indiana recently contracted measles, including one who took part in Super Bowl festivities on Feb 3, and two others are listed as having probable cases, the Indiana State Department of Health (ISDH) confirmed yesterday. The confirmed case-patients are from Hamilton County and the probable cases are in Boone County, the agency said in a news release. One of the patients with a confirmed case visited the outdoor Super Bowl Village Feb 3, which drew 200,000 people that day, the Associated Press (AP) reported yesterday. The patient did not go inside the Convention Center for the NFL Experience, the ISDH said. He or she contracted the disease from an undiagnosed sibling, according to the AP, which cited the ISDH. The release said the agency has contacted the US Centers for Disease Control and Prevention about the cases, as well as health officials in New York and Massachusetts, the home states of the two
Super Bowl teams, the New York Giants and New England Patriots.
Feb 8 ISDH news release