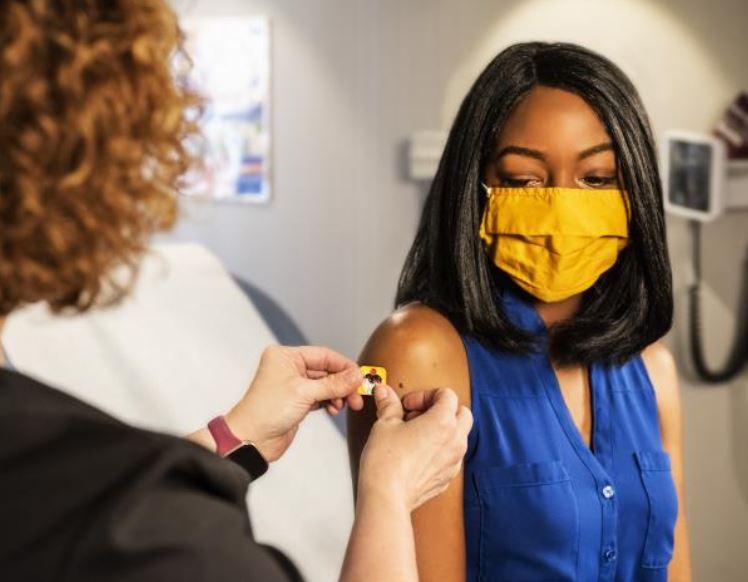
An independent vaccine advisory group to the Centers for Disease Control and Prevention (CDC) today, in an emergency session, recommended that Americans ages 18 and older receive the Moderna COVID-19 vaccine under emergency use.
The move—like its action last week for the Pfizer-BioNTech vaccine—paves the way for healthcare workers to begin immunizing people.
The CDC typically accepts the recommendations of its Advisory Committee on Immunization Practices (ACIP), and a formal announcement from the CDC will be the final step for vaccination with the second vaccine to begin. Moderna vaccine shipments are already under way, following yesterday's emergency use authorization (EUA) announcement by the Food and Drug Administration (FDA; see related CIDRAP story).
The vote from ACIP passed with 11 in favor and 3 recusals because of conflicts of interest.
Lynn Bahta, RN, MPH, with the Minnesota Department of Health, said she voted yes, because adding a second vaccine has the potential to be live-saving, with a "horrendous" number of daily deaths reported, despite the fact that there's still a lot to learn about the disease and the vaccine.
What's known about anaphylaxis
A key theme in the discussions today were concerns about anaphylaxis reactions. Tom Clark, MD, MPH, deputy director of the division of viral disease in the CDC's National Center for Immunization and Respiratory Diseases, had more details about such events in the United States immediately following Pfizer-BioNTech vaccination.
So far, 6 instances have been reported among an estimated 272,001 doses administered so far. One was in an individual who had an earlier reaction after rabies vaccination. A few days ago, the CDC posted interim recommendations on recognizing and managing potential anaphylaxis at COVID-19 vaccination sites.
Though two cases were in Alaska, there's no obvious geographic clustering, nor was a specific production lot involved. The ACIP has said that people who experience anaphylaxis after the first dose shouldn't receive a second dose of COVID-19 vaccine.
Questions on variant virus, specific populations
Moderna representatives also responded to concerns about reports of faster-spreading variants identified in the United Kingdom, one Canadian province, and South Africa. They said the company has been looking at mutated strains and has been testing them on animal sera, and so far, they haven't seen any problems with vaccine response.
Moderna officials said researchers will also conduct similar experiments with human sera and initiate deep genetic sequencing on SARS-CoV-2 samples from vaccinated patients who get sick with COVID-19.
Regarding study of the vaccine in children, the company said it began a trial among adolescents ages 12 to 18 on Dec 9 and that results are expected in 2021.
ACIP members also had questions about vaccination in pregnancy. Moderna has said it is putting together a registry of people in clinical trials who were pregnant or became pregnant, and Clark said today that surveillance has identified that 514 pregnant women have received the Pfizer-BioNTech vaccine since its rollout.
Tomorrow, ACIP meets in another emergency session to recommend the next priority groups to receive COVID-19 vaccines. Currently, healthcare workers and nursing home residents are the only groups prioritized to receive them.