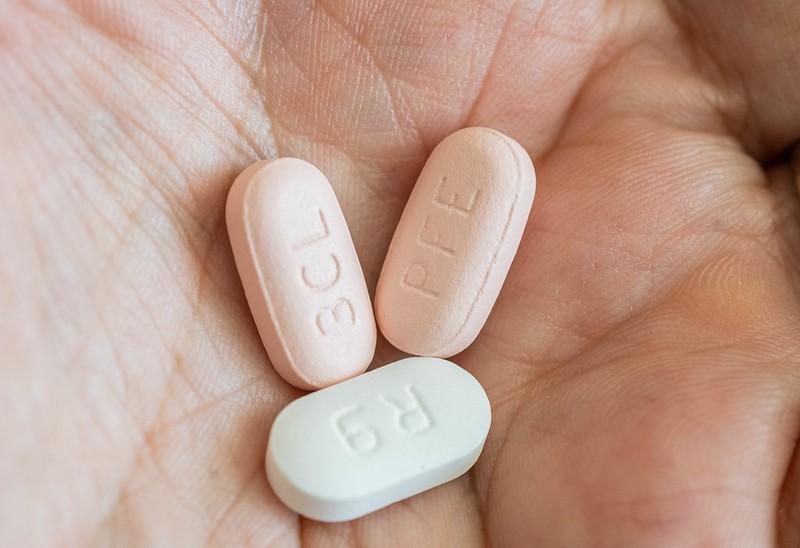
Today in JAMA Internal Medicine researchers from the National Library of Medicine and the National Institutes of Health describe a small reduction in post-COVID condition (PCC or long COVID) among US adults ages 65 and older who were treated with either the antiviral drug nirmatrelvir (Paxlovid) or molnupiravir (Lagevrio).
The studies were based on Medicare enrollees diagnosed as having COVID-19 from January to September 2022. Prescription of nirmatrelvir or molnupiravir was considered to be indicative of COVID-19 infection, as at-home testing was widely available during the study period.
PCC was defined as any new occurrence (not present prior to COVID-19 diagnosis) of 11 noted symptoms from 4 to 12 weeks after infection, including fatigue, difficulty breathing, heart palpitations, and memory problems.
Antivirals less protective in women
In a cohort of 313,262 participants, 51,658 took nirmatrelvir during acute infection, and 8,089 took molnupiravir. PCC incidence was 11.8% among patients receiving nirmatrelvir, 13.7% for molnupiravir, and 14.5% for neither. The absolute risk reduction was 2.7% for nirmatrelvir and 0.8% for molnupiravir.
The effect was smaller among females, Asian, Black, and Hispanic race, and patients with lower incomes. The hazard ratio for females compared to males was 0.89 compared to 0.84 for nirmatrelvir, and 0.95 compared to 0.88 for molnupiravir.
"The current approved use of the 2 drugs is for the prevention of severe acute COVID-19," the authors wrote. "Our findings suggest that they may also have a role in preventing PCC."
Our findings suggest that they may also have a role in preventing PCC.
Notably, the authors said vaccination status was not included in any final analysis. Though the study showed a smaller benefit to the approved antivirals than previous studies, it is one of the largest studies to look at the effect of the drugs on PCC.















