May 18, 2012
Salmonella sushi outbreak tops 300 cases
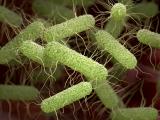
The number of people infected with Salmonella Bareilly or Salmonella Nchanga from eating raw yellowfin tuna has risen to 316 in 26 states, with 304 of those cases linked to the Bareilly strain, the US Centers for Disease Control and Prevention (CDC) said yesterday. That's an increase of 58 cases since the last CDC outbreak update, on May 2. Of all patients, 37 (12%) have been hospitalized, but no deaths have been reported. States with 25 or more cases are New York (54), Massachusetts (33), New Jersey (28), Maryland (27), Illinois (27), and Pennsylvania (25). Trace-back investigation has determined the cases to stem from consuming frozen raw yellowfin tuna product, known as Nakaochi Scrape, from Moon Marine USA Corp. of Cupertino, Calif. Testing in several states has isolated Salmonella from 53 of 55 samples taken from intact packages of the product or from sushi prepared with it, the CDC said.
May 17 CDC update
FDA takes South Korean shellfish off the market
In a constituent update today to distributors, retailers, and food service operators, federal officials said shellfish from South Korea should be removed from the market. The US Food and Drug Administration's (FDA's) Center for Food Safety and Applied Nutrition (CFSAN) said oysters, clams, mussels, and scallops harvested from South Korea waters "may have been exposed to human fecal waste and have the potential to be contaminated with norovirus," although no US illnesses have been linked to the shellfish this year. The update said the FDA removed the shellfish from the Interstate Certified Shellfish Shippers List following a comprehensive FDA evaluation that determined "the Korean Shellfish Sanitation Program no longer meets the sanitation controls spelled out under the National Shellfish Sanitation Program." The agency found several deficiencies in the program, including poor management of pollution sources, inadequate sanitary controls
for preventing human waste from entering fish farms, and detection of norovirus in shellfish-growing areas.
May 18 CFSAN constituent update on Korean shellfish
FDA announces public meeting on international food safety steps
In a separate constituent update today, CFSAN announced a public meeting in June on the FDA Food Safety Modernization Act that will deal with international capacity building. The meeting will be held Jun 19 from 9:00 am to 5:00 pm EST at the L'Enfant Plaza Hotel in Washington, D.C. In the update, CFSAN said the meeting is designed to provide discussion on the FDA's "comprehensive plan to expand the technical, scientific, and regulatory capacity of foreign governments and their respective food industries in countries that export foods to the United States." The public will have an opportunity to provide input on the process, the agency said.
May 18 CFSAN constituent update on public meeting
Public meeting registration page