Oct 24, 2012 (CIDRAP News) – Massachusetts officials yesterday said New England Compounding Center (NECC), the firm linked to a multistate fungal meningitis outbreak, sent shipments from two of the recalled lots before it received sterility testing results.
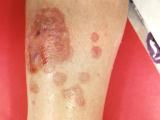
Investigators also found black particulate matter in several unopened vials of the recalled methylprednisolone acetate, which was used in epidural injections for back pain and injections for peripheral joint problems, according to a report of preliminary investigation findings.
Federal officials have said that as many as 14,000 patients could have been exposed to the recalled injections.
Nine more infections have been reported in the outbreak, raising the number of infections to 317, the US Centers for Disease Control and Prevention (CDC) said today in its latest update. One more death was reported, putting that total at 24, and the number of affected states held steady at 17.
The CDC reported one more joint infection linked to the contaminated steroids, raising that number to five, and it said 54 cases have now been confirmed with lab tests. So far all but two of the infections involve Exserohilum rostratum, a type of black mold that hadn't previously been known to cause meningitis.
In a press conference with reporters yesterday, Madeleine Biondolillo, MD, who directs the Massachusetts Department of Public Health's Bureau of Health Care Safety and Quality, said the investigation began on Sep 25 and involves collaboration with their colleagues at the US Food and Drug Administration (FDA) since Oct 1. She said the investigation is ongoing and that authorities have obtained evidence, reviewed NECC's standard operating procedures, probed the company's records, and interviewed its employees.
NECC didn't label the medications for specific patients, a violation of Massachusetts licensing regulations for compounding pharmacies, Biondolillo said.
According to the Massachusetts Department of Public Health (MDPH) investigation report, NECC's sterility tests on the shipments sent before results came in didn't show contamination. However, investigators said they were still exploring the adequacy of NECC's testing methods.
In addition, authorities found that the company didn't follow established standards for autoclaving and didn't keep products in the autoclave for the minimum 20 minutes needed to ensure sterility.
The report also says NECC didn't thoroughly clean powder hoods used to protect pharmacists while they prepare compounded substances. Investigators observed residual powder within the hood, which could lead to contamination of medications.
Mats designed to trap dirt and other contaminants were visibly soiled, and a leaking boiler next to the clean room caused water to pool around it and adjacent walls, creating conditions that could support contaminant growth, according to the findings.
At the media briefing, Massachusetts governor Deval Patrick said federal officials have launched a criminal investigation into NECC's actions and he announced more steps the state and its Board of Pharmacy have taken in response to the steroid contamination, including permanently revoking NECC's operating license and those of its three principal pharmacists.
"Those whose laboratory practices caused this outbreak should never practice pharmacy or manufacture in Massachusetts again," he said.
Patrick said that, over the years, some pharmacies have evolved from neighborhood drug stores to large-scale manufacturers that sell products across state lines, and that regulations in Massachusetts and at the federal level have not kept pace with the changes. He said that he had directed the pharmacy board to start making unannounced inspections of compounding pharmacies that make sterile injectable medications and strengthen penalties for pharmacies that don't comply with rules.
The state will now require compounding pharmacies in Massachusetts to submit annual production, volume, and distribution reports to make it easier for authorities to flag operations that are acting more like manufacturers, which require federal licensing and extra scrutiny, Patrick said. He added that the state will require compounding pharmacies to report interactions with federal regulators and will form a special commission to examine best practices in other states and what changes may be needed at the federal level.
In a related development, two members of Congress yesterday asked the Government Accountability Office (GAO) to investigate compounding pharmacies and their oversight by state and federal agencies. The members are Rep John Tierney, D-Mass., ranking member of the House Subcommittee on National Security, Homeland Defense, and Foreign Operations, and Rep Elijah Cummings. D-Md., ranking member on the House Committee on Oversight and Government Reform.
In a statement outlining their letter to the GAO, Tierney said, "In light of these tragic deaths, we need to know how many other companies are engaged in similar activity and whether they may be falling through the cracks between state and federal regulating agencies."
Meanwhile, the CDC yesterday issued a new health alert network advisory to clinicians that contained its latest advice on how to manage asymptomatic patients who received epidural or paraspinal injections with the contaminated steroids.
The agency still advises against prophylactic antifungal treatment for people exposed to the recalled medication in the absence of diagnostic tests that suggest meningitis. The antifungal drugs used to treat the condition require powerful doses that can cause serious adverse side effect.
Based on new data, however, the CDC said the period of greatest risk for developing fungal meningitis is during the first 6 weeks (42 days) after injection, so clinicians should consider additional monitoring.
The FDA has reposted new lists of customers who received the recalled steroids from NECC, after taking them down upon finding technical problems with them. The agency first posted the lists on Oct 22, with the caveat that the information was from NECC and it couldn't guarantee their accuracy or completeness.
See also:
Oct 23 MDPH NECC preliminary investigation report
Oct 24 CDC fungal meningitis outbreak update
Oct 23 Biondolillo comments
Oct 23 Patrick comments
Oct 23 Rep Tierney statement
Oct 23 CDC HAN health advisory
Oct 22 FDA statement and lists