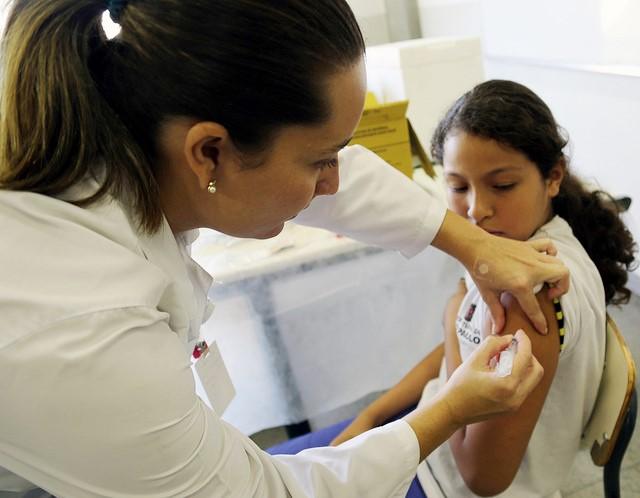
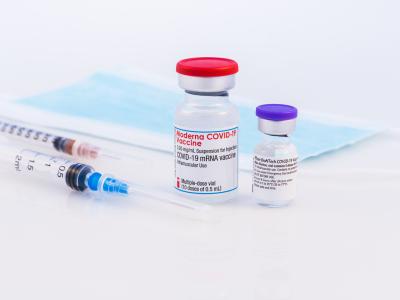
A large double-blind study conducted in 18 countries found the nine-valent (nine-strain) human papillomavirus (HPV) vaccine offered long-term protection against most viruses that cause cervical cancers for girls and women ages 16 to 26 years old.
The study, published yesterday in the TheLancet, updates and confirms the phase 3 findings of the vaccine, Gardasil 9, first published in 2015.
In this study, researchers recruited 14,215 women at 105 study sites around the world to receive either the nine-valent HPV vaccine or the quadrivalent (four-strain) HPV vaccine (Gardasil). The participants received three doses of the vaccine over the course of 6 months. The girls and women recruited for the study were healthy, had no previous history of abnormal cervical changes, and had had fewer than four sexual partners.
The investigators found a 97.4% efficacy for Gardasil 9 against high-grade cervical, vulvar, and vaginal disease related to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58. The nine-valent HPV vaccine conferred the same levels of immunity as the quadrivalent vaccine against HPV types 6, 11, 16, and 18, the strains contained in Gardasil that cause 70% of cervical cancers and most genital warts.
The four- and nine-valent vaccines had similar safety profiles. Gardasil 9 was approved by the US Food and Drug Administration in 2014, and Gardasil in 2006.
Promise against cervical cancer
While the vaccine is an unequivocal success, the challenge will be implementing its use, the authors of the study said.
"Nationwide, 40 percent of girls and boys do not receive the HPV vaccine," said the study's primary author, Warner Huh, MD, director of the University of Alabama at Birmingham Division of Gynecologic Oncology, in a university press release.
Huh said Gardasil 9 offers protection against 90% of the HPV strains that cause cervical cancer. If widely used, the vaccine could help wipe out cervical cancer, he added.
"Looking forward, with widespread vaccination, it is highly likely that cervical cancer will evolve into historical interest only, and screening, like Pap smears, might go away altogether," said Huh. "HPV vaccines are one of the most scrutinized vaccines ever, but multiple studies have demonstrated the vaccine to be safe and well-tolerated."
Currently, the nine-valent vaccine is licensed in 60 countries as a prophylactic measure against cervical cancer and genital warts. But an accompanying commentary in the The Lancet penned by South African gynecologist Lynette Denny, MD, PhD, warned that the vaccine is unlikely to make a difference in the lives of women in low- and middle-income countries (LMICs), where routine cervical screening is uncommon.
"It is essential that the international community pay attention to the gross inequities that exist in cervical cancer prevention efforts,and although the research presented in this paper is robust, credible, and done to the highest standards, it might still be far from providing an intervention that will save the lives of women in LMICs."
See also:
Sep 5 Lancet study
Sep 5 University of Alabama press release
Sep 5 Lancet commentary