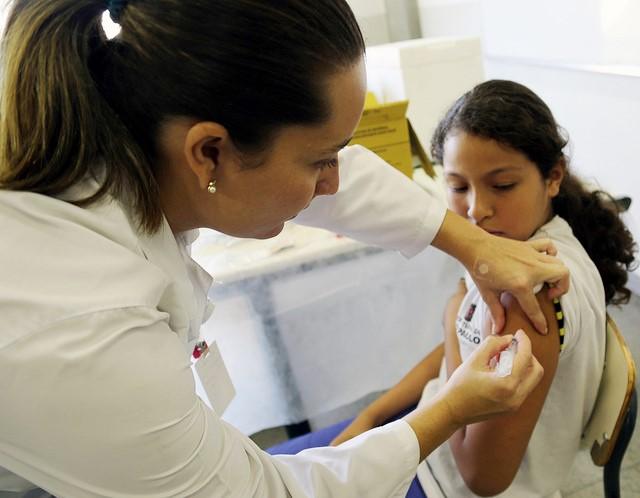
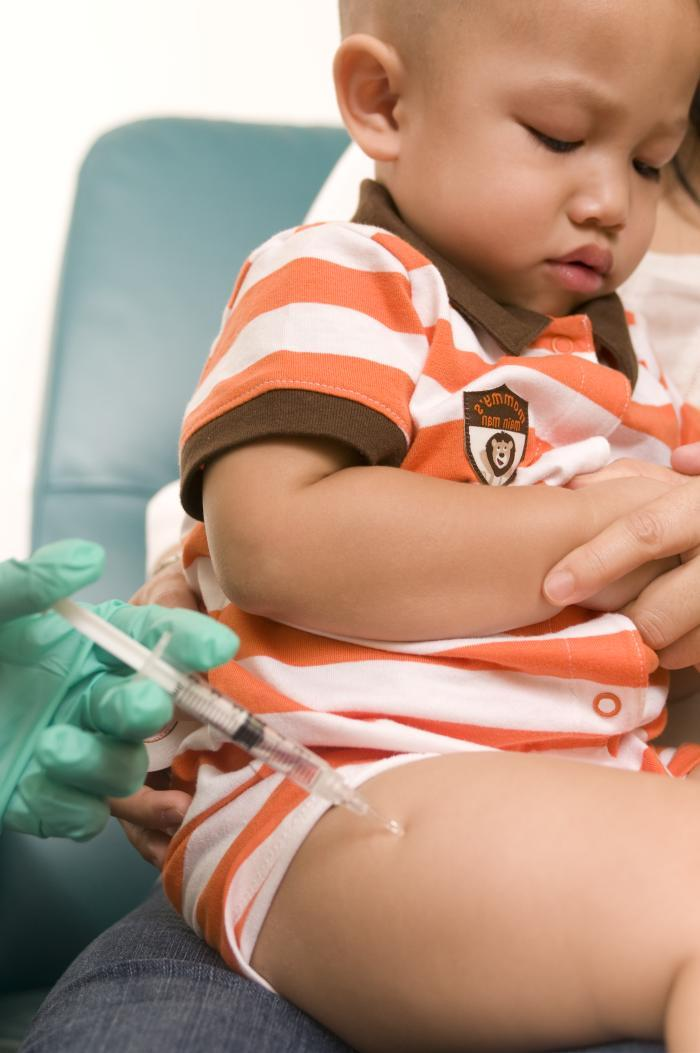
In a meeting yesterday to discuss the strains to include in flu vaccines for the 2024-25 flu season, a federal vaccine advisory group urged manufacturers of vaccines for the US market to drop the influenza B Yamagata lineage strain.
The Food and Drug Administration (FDA) Vaccines and Related Biological Products Advisory Committee (VRBPAC) in an announcement following the meeting noted that the Yamagata influenza B lineage hasn't been detected since March 2020, and evidence suggests it no longer poses a public health threat.
Move is part of broader push for return to trivalent formulations
Yesterday's vote on a return to trivalent (three-strain) vaccines follows its discussions in October 2023 about whether quadrivalent (four-strain) vaccines were still needed. In September 2023, the World Health Organization (WHO) flu vaccine advisers recommended a switch back to trivalent flu vaccines.
The FDA said it has been working with manufacturers to move to trivalent formulations for the upcoming flu season. "FDA anticipates that there will be an adequate and diverse supply of approved trivalent seasonal influenza vaccines for the United States in the coming season," it said.
FDA anticipates that there will be an adequate and diverse supply of approved trivalent seasonal influenza vaccines.
However, it also acknowledged that not all countries can revert to trivalent vaccines as fast as the United States is moving and that companies that have existing FDA-approved flu vaccines and want to export them can include the Yamagata influenza B strain.
VRBPAC also approved the strains to include in the 2024-25 trivalent vaccines, which align with the ones recommended for the upcoming Northern Hemisphere season by the World Health Organization for both egg- and cell-based vaccines.