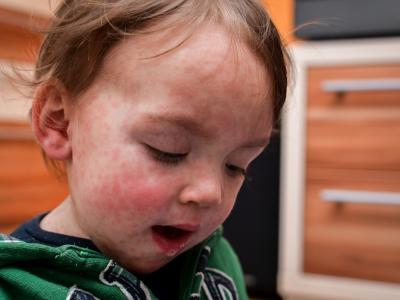
Long COVID relatively rare in children and teens, study suggests
A study published yesterday in JAMA Pediatrics suggests that long COVID is uncommon in children and teens and that risk factors include severe SARS-CoV-2 infection, younger age, and complex underlying chronic diseases.
The US Centers for Disease Control and Prevention defines long COVID as continuous, relapsing, or new symptoms or conditions persisting at least 1 month after the initial infection.
University of Colorado researchers evaluated the PEDSnet electronic health records (EHRs) of 659,286 patients aged 0 to 20 years tested for COVID-19 at one of nine US hospitals from Mar 1, 2020, to Oct 31, 2021. Average follow-up time was 4.65 months. The average patient age was 8.1 years, 52.8% were male, and 9.1% tested positive for COVID-19. The hospitals were located in Pennsylvania, Ohio, Colorado, Illinois, Delaware, Florida, Washington state, and California.
COVID-19 patients had elevated rates of myocarditis (inflammation of the heart muscle; adjusted hazard ratio [aHR], 3.10), acute respiratory distress syndrome (aHR, 2.96), muscle inflammation (aHR, 2.59), other or ill-defined heart disease (aHR, 1.47), fluid or electrolyte disturbances (aHR, 1.45), treatment for mental illness (aHR, 1.62), and anxiety (aHR, 1.29).
Other common conditions included changes in smell and taste (aHR, 1.96), hair loss (aHR, 1.58), chest pain (aHR, 1.52), abnormal liver enzymes (aHR, 1.50), rashes (aHR, 1.26), fatigue and malaise (aHR, 1.24), fever and chills (aHR, 1.22), cardiorespiratory signs and symptoms (aHR, 1.20), and diarrhea (aHR, 1.18).
A total of 41.9% of COVID-positive patients reported at least one systemic or syndromic long-COVID sign or symptom or use of an over-the-counter cough and cold medication, compared with 38.2% among test-negative patients (difference, 3.7 percentage points, or a 9.7% higher incidence).
Risk factors for long COVID included a stay in an intensive care unit during infection, age younger than 5 years, and complex chronic conditions.
"Our findings suggest that the burden and risk windows of PASC [postacute sequelae of SARS-CoV-2] may differ between children and adults," the researchers wrote. "Future studies, including long-term prospective studies, such as the National Institutes of Health RECOVER Initiative, are needed to fully elucidate PASC phenotypes."
Aug 22 JAMA Pediatr study
Olympics study shows wastewater sampling useful for COVID-19 outbreaks
As part of precautions taken during the 2020 Olympic and Paralympic Games in Tokyo, held in August and September of 2021, researchers tested wastewater in the sewage system of the Olympic Village daily, as well as athletes, for SARS-CoV-2. Today in JAMA Network Open, the authors show that the two practices complemented each other, and wastewater-based epidemiology (WBE) could predict uptick in COVID-19 cases.
The study compared clinically reported cases and viral loads in wastewater by using 360 samples collected from manholes in 7 areas of the Olympic Village to reported COVID-19 cases as given to the Tokyo 2020 Organizing Committee.
SARS-CoV-2 was found in 151 wastewater samples, including 98 during the Paralympics and 53 during the Olympics. The authors found a correlation between positive wastewater and clinical case positives, with positive sewage samples appearing 2 to 3 days before an uptick in clinically positive cases.
The Paralympics had more cases compared with the Olympics: 3.6 versus 3.2 confirmed cases per 1,000 participants.
"These findings suggest that WBE and clinical tests are complementary and that the testing strategy played a role in preventing COVID-19 clusters in the village. This study of one of the world’s largest mass gatherings provides novel evidence on the implementation and use of WBE in communities where all members undergo daily testing," the authors concluded.
Aug 22 JAMA Netw Open study
Global flu activity declining in most of world, except Southeast Asia
In an update that covers the last week of July and the first week of August, the World Health Organization (WHO) said yesterday that flu activity is declining in most parts of the world, except for in Southeast Asia.
Cambodia, Malaysia, Singapore, and Thailand are among the countries in Southeast Asia that are reporting rising flu activity, mostly involving H3N2. In East Asia, flu activity appears to have peaked in southern China, where H3N2 also predominated.
Meanwhile, in southern Asia, India reported a rise in H1N1 detections.
In the Southern Hemisphere, downward trends were reported from Australia and New Zealand, while levels remained stable in South Africa and declined in temperate parts of South America. In tropical African nations, flu activity declined. In North America and Europe, flu remained at inter-seasonal levels.
Globally, of respiratory samples that tested positive for flu during the latest reporting period, 96.2% were influenza A, and of the subtyped influenza A samples, 93.8% were H3N2.
Aug 22 WHO global flu update
Avian flu strikes more flocks in 3 states
Avian flu outbreaks continue over the summer months in US poultry flocks, with three more outbreaks reported, according to the latest updates from the US Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS).
One of the outbreaks struck a commercial broiler breeding farm housing 33,900 birds in Fresno County, California. Another affected backyard birds at a location in Washington's Kitsap County.
Also, the virus struck an animal rescue/rehabilitation facility in Henry County, Georgia, which has 90 birds. The Georgia Department of Agriculture said the outbreak is the second in Georgia and that the flock owners reported deaths in wild black vultures, as well illnesses in domestic birds. Samples were positive for highly pathogenic avian influenza.
Since the H5N1 outbreaks began in early February, the virus has led to the loss of 40.1 million birds across 39 states. Detections in wild birds have also continued over the summer in multiple US regions.
USDA APHIS poultry outbreak updates
Georgia Department of Agriculture update