Rapid, point-of-care test for respiratory infections could cut antibiotic use
A study today in the journal Family Practice reports that a rapid, multi-viral point-of-care test for respiratory infections was easy to use, acceptable to patients and clinicians, and appeared to influence clinical reasoning about antibiotics.
In the study, clinicians at four general practitioner (GP) practices in South West England used the BioFire Filmarray v1.7 test, which can detect 17 different types of respiratory viruses and three atypical respiratory bacteria from nose and throat swabs in around 65 minutes, on a sample of 93 patients older than 3 months who had suspected acute respiratory infections. Seventy-two percent of the samples were processed in less than 4 hours, and 90% in less than 24 hours. The median set-up time for each test was 2.7 minutes.
Of the 93 nose and throat swabs tested, 37% had no pathogens, 58% had at least one virus, and 2% had atypical bacteria. Pre-test, antibiotics were prescribed to 35% of patients with no pathogen detected and 25% of patients with a virus. Post-test diagnoses changed in 20% of patients, and the proportion of patients in whom clinicians' diagnoses were certain or very certain increased after test use, particularly when a pathogen was detected.
Clinicians were also less likely to predict the patient would benefit from antibiotics post-test. Less than 10% of patients, however, were contacted after testing to change their treatment plan.
Interviews with clinicians, test processors, and administrators revealed that the test was easy to run and that clinicians liked it, but wanted quicker results and wanted more clinically relevant bacteria to be included. Overall, clinicians indicated they would reserve judgment until the test's ability to support clinical management, improve outcomes, and reduce antibiotic use could be demonstrated in clinical trials.
"This was a small-scale feasibility study, and clinical trials are now needed to see if these point-of-care tests can safely and cost-effectively reduce antibiotic prescribing in primary care," lead study author Alastair Hay, MD, a professor at the University of Bristol's Centre for Academic Primary Care, said in a university press release.
Mar 4 Fam Pract abstract
Mar 4 University of Bristol press release
Global flu A and B activity stays mainly sporadic
Global flu activity remained mostly sporadic, even though many countries are looking closely for it in the context of the COVID-19 pandemic, the World Health Organization (WHO) said in its latest regular flu update, which covers the first half of February.
A handful of Middle East countries reported sporadic influenza B and H3N2 cases, with influenza B detected at low levels in southern China.
In tropical regions, West African countries Ivory Coast and Togo reported influenza A activity, and in middle Africa, Cameroon reported influenza B and the Democratic Republic of Congo (DRC) reported H3N2 over the past few weeks. In eastern Africa, Ethiopia recently reported H3N2 and influenza B. India has reported sporadic H3N2 cases, and in Southeast Asia, some countries such as Laos and Thailand reported H3N2 activity.
At the global level, of the few respiratory specimens that tested positive for flu, 64.1% were influenza B. And of the subtyped influenza A viruses, 52.4% were 2009 H1N1 and 47.6% were H3N2.
Mar 1 WHO global flu update
West African countries coordinate response to Guinea Ebola outbreak
In the latest Ebola outbreak developments, health ministers from West Africa met on Mar 3 and agreed on a plan to coordinate their response to Guinea's recent outbreak, the first in the region since the massive outbreak of 2014-16.
Aside from Guinea, countries that were part of talks include Ivory Coast, Guinea-Bissau, Liberia, Mali, Senegal, and Sierra Leone.
The WHO said in a statement yesterday from its African regional office that in addition to efforts to contain the virus, the countries agreed to coordinate import regulations for vaccines and drugs and promote the steps that brought the region's earlier outbreak under control.
Guinea's current outbreak total remains at 17 cases, of which 13 are confirmed, and 8 deaths, according to the latest update from the WHO.
In a related development, the United Nations International Organization for Migration (IOM) said today that it has issued an appeal for $8 million to support response efforts in Guinea's outbreak at both the prefecture level and at key country border crossings. It said it has already set up five screening points on Gouecke, where Guinea's recent outbreak began, and on the borders with Ivory Coast and Liberia. The group also said it has activated three emergency operations centers.
In the Democratic Republic of the Congo (DRC) outbreak, no new cases have been reported, keeping the total at 11 cases, 4 of them fatal, according to the latest update from the WHO.
Mar 3 WHO African regional office statement
Mar 4 WHO African regional office case count tweet
Mar 4 IOM statement
Mar 2 WHO African regional office DRC update tweet
Lower rotavirus vaccine price for humanitarian use announced
The WHO and three of its partners—Doctors Without Borders (MSF), Save the Children, and UNICEF—today announced that they have reached a pricing agreement with pharmaceutical company GSK to make rotavirus vaccine available to more children in humanitarian crisis settings.
The groups said the lower priced vaccine will allow children in refugee camps, displaced communities, and other emergency situations a better chance of being protected against severe diarrheal disease.
The agreement marks the second use of the Humanitarian Mechanism, which was launched in 2017 with lower priced pneumococcal vaccine, of which nearly 1 million doses have been approved for countries that need it.
In a WHO statement, Kate O'Brien, MD, MPH, the WHO's director of immunization, vaccines, and biologicals, said " We welcome this engagement from manufacturers and hope it will be a step towards making more vaccines available in the future at affordable prices." Rotavirus, the most common cause of severe diarrheal disease in kids younger than 5, is linked to nearly 200,000 deaths each year, many of them in refugee camps and other settings where conditions are crowded and hygiene and sanitation are poor.
GSK said in a statement that it has committed to supply Rotarix at its lowest global price for the tube presentation, which is already supplied to UNICEF. Thomas Breuer, MD, GSK's chief medical officer, said, "We are delighted to become the first company to offer a rotavirus vaccine through the Humanitarian Mechanism for use with some of the children most vulnerable to severe diarrheal disease."
Mar 4 WHO statement
Mar 4 GSK statement
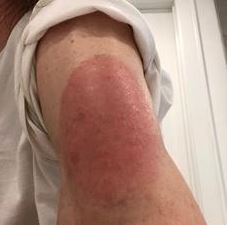 p to 10 centimeters (4 inches) in diameter (see photo at right, used with permission of Massachusetts General Hospital). Some concurrent systemic conditions also occurred, such as high blood pressure, fatigue, additional rashes, and fever. Median onset was day 8 post-vaccination (range, 4 to 11) and the reactions cleared in a median of 6 days.
p to 10 centimeters (4 inches) in diameter (see photo at right, used with permission of Massachusetts General Hospital). Some concurrent systemic conditions also occurred, such as high blood pressure, fatigue, additional rashes, and fever. Median onset was day 8 post-vaccination (range, 4 to 11) and the reactions cleared in a median of 6 days.












