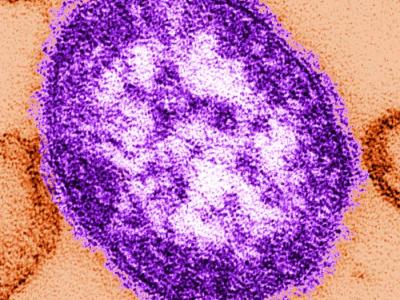
Pfizer today announced that its respiratory syncytial virus (RSV) vaccine Abrysvo maintained high efficacy in the second season after vaccination in adults ages 60 and older, according to the latest data from an ongoing phase 3 clinical trial.
In a press release, the company said vaccine efficacy against RSV-related lower respiratory tract disease, defined by three or more symptoms, after season two was 77.8% (95.0% confidence interval [CI], 51.4% to 91.1%). For comparison, vaccine efficacy after season one was 88.9% (95% CI, 53.6% to 98.7%).
Efficacy was consistent across the seasons for both RSV A and RSV B strains. Researchers also saw sustained efficacy against less severe symptoms: 65.1% after season one and 55.7% after season two. No new adverse events were reported through the second season.
Durable protection against 2 strains
Annaliesa Anderson, PhD, senior vice president and chief scientific officer of vaccine research and development at Pfizer, said the company is encouraged by the level of protection after two full RSV seasons. "This new data indicate that broad and durable protection against both types of RSV that cause disease, RSV A and RSV B, is the potential benefit to having a bivalent vaccine."
Broad and durable protection against both types of RSV that cause disease, RSV A and RSV B, is the potential benefit to having a bivalent vaccine.
The company said it will submit the data to regulatory authorities and vaccine technical committees and will publish the findings in a peer-reviewed scientific journal.
In the United States, the Food and Drug Administration (FDA) approved Abrysvo for people ages 60 and older in May 2023, and the following month the Centers for Disease Control and Prevention recommended Abrysvo and the RSV vaccine made by GSK (Arexvy) for adults ages 60 and older based on shared clinical decision making with health providers. In September, the CDC recommended that pregnant women receive Abrysvo as a strategy to protect newborns from RSV.