Dec 9, 2009
BARDA shifts approach for developing new anthrax vaccine
The Biomedical Research and Development Authority (BARDA), part of the US Department of Health and Human Services (HHS) said on Dec 7 that it is changing its approach for supporting the development of a next-generation anthrax vaccine. The announcement came as BARDA canceled its request for proposal for companies to develop a recombinant protective-antigen anthrax vaccine for the Strategic National Stockpile, after its technical panel determined none of the companies could meet the Project BioShield requirement to have a product licensed within 8 years. Instead, BARDA is asking vaccine developers to submit plans under a program that more broadly supports countermeasure development. BARDA said studies funded through this program can help advance vaccines far enough to improve their chances of meeting Project BioShield requirements in the future. Dr. Robin Robinson, BARDA director, said the earlier proposal would expose companies
to an undue risk of failure. He said anthrax preparedness remains one of the nation's highest priorities. The government has sought a more modern anthrax vaccine because a number of military members, who are required to get the shot, have complained of serious side effects. In 2006 BARDA canceled a contract with VaxGen to produce a new anthrax vaccine after problems with vaccine stability caused the company to miss its clinical trial launch deadline.
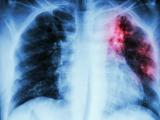
WHO reports good news in global TB battle
Over the past 15 years a rigorous approach to tuberculosis (TB) treatment has cured 36 million people and saved about 8 million lives, the World Health Organization (WHO) said yesterday in its latest TB report. In 1994 the WHO incorporated the DOTS strategy into its TB program, which added five elements: political commitment with sustained financing, case detection through quality bacteriology, standardized treatment with supervision and patient support, an effective drug supply system, monitoring and evaluation protocols, and impact measurement. In the past year, 87% of treated patients were cured, marking the first time the global target of 85% has been met since it was set in 1991, the WHO said. Progress is being made on addressing TB and HIV coinfections, but not all patients are receiving the treatment they should. Multidrug-resistant TB and extensively drug-resistant TB are still a persistent challenge in many parts of the world, the
agency reported.
Dec 8 WHO press release
WHO TB report