Jun 11, 2012
ECDC report addresses drug-resistant gonorrhea
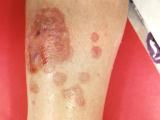
With drug-resistant gonorrhea increasing in Europe, the European Centre for Disease Prevention and Control (ECDC) today released a plan to increase surveillance and testing, improve diagnosis and treatment, address treatment failure, and raise awareness. ECDC data show that the percentage of gonorrhea isolates with decreased cefixime susceptibility (the drug of choice) increased from 4% in 2009 to 9% in 2010. In response, ECDC Director Marc Sprenger, MD, PhD, said in a news release today, "Decreasing susceptibility to recommended antimicrobials and increasing numbers of treatment failures across Europe ask for careful monitoring of the European gonococcal population as the loss of the current recommended treatments could result in untreatable gonorrhoea." He added, "Only continued surveillance of antimicrobial resistance in gonococci will inform existing and future treatment guidelines adequately in order to ensure that patients are treated with effective drugs and morbidity is reduced in infected patients." He also said European treatment guidelines need to be reviewed. Last week the World Health Organization (WHO) also issued a warning on drug-resistant gonorrhea.
Jun 11 ECDC news release
Jun 11 ECDC full report
Jun 6 CIDRAP News Scan on WHO alert
Shortage causes Nunavut to ration TB vaccine
Nunavut territory in northern Canada is rationing tuberculosis (TB) vaccine after drug maker Sanofi Pasteur stopped distributing it 2 months ago because of production problems, CBC News reported. Normally all newborns in the territory receive Sanofi's BCG vaccine because the region has a TB rate 75 times the national average. But, with only 40 vaccine vials left, health officials are pacing vaccinations. Each vial contains 10 doses, but they must be used within 8 hours of opening the vial, so the plan is to wait until 10 babies are born before vaccinating them en masse rather than individually right after birth. "It may be a few months before we're able to get it to every child, but we don't expect there to be any adverse effects on their child due to this supply issue," said Maureen Baikie, MD, Nunavut's deputy chief medical officer. Sanofi said it is experiencing problems in the final vaccine test stage at its Ontario plant and the timetable for resolution is uncertain.
Jun 8 CBC News story
CDC devotes 80 to 90 employees daily to global anti-polio efforts
Over the past half year, the US Centers for Disease Control and Prevention (CDC) has mobilized 350 staff members to help in the global war against polio and averages 80 to 90 employees each day involved in such efforts, the agency said last week. CDC Director Thomas R. Frieden, MD, MPH, activated the CDC's Emergency Operations Center (EOC) on Dec 2 to strengthen the agency's polio response with global partners via the Global Polio Eradication Initiative (GPEI). EOC activation has enhanced capacity for the CDC's Stop Transmission of Polio program, which trains public health volunteers (75 since Dec 2) to improve polio surveillance and vaccination campaigns. The EOC has also helped scale up in-country technical expertise, according to the CDC, as well as assisted with program evaluation, data dissemination, and other collaborative efforts. Afghanistan, Nigeria, and Pakistan remain the world's only polio-endemic nations, but the disease has resurged in other countries.
Jun 8 CDC update
Foot-and-mouth disease vaccine gets conditional USDA license
A foot-and-mouth disease (FMD) vaccine developed by two private companies in partnership with the federal government has received a conditional license from the US Department of Agriculture (USDA), the companies announced last week. The product, an adenovirus engineered to express certain FMD proteins, is the first US-made FMD vaccine for cattle, according to Benchmark Biolabs of Lincoln, Neb., which collaborated on the vaccine with GenVec Inc., of Gaithersburg, Md.; the Department of Homeland Security; and the USDA's Plum Island Animal Disease Center. The conditional license means USDA officials can authorize distribution of the vaccine in an emergency, according to a GenVec statement. The company said the license was issued in recognition of the need for an FMD vaccine that can be made in the United States and allows for differentiating between infected and vaccinated animals. FMD is an economically devastating animal disease, and handling live FMD viruses has long been illegal in the United States, except at Plum Island. The new vaccine can be made without having to handle live FMD virus, according to Benchmark Biolabs. "The vaccine candidate was developed to be able to survive injection and immunize animals but to be incapable of longer-term survival outside a laboratory or production plant setting," the company said. The United States has not had an FMD outbreak since 1929. The USDA plan for responding to an outbreak has relied on obtaining a vaccine from foreign manufacturers, if vaccination was deemed necessary.
Benchmark Biolabs press release
Jun 7 GenVec press release
USDA information on FMD vaccines