Data from a phase 2 clinical trial show promising results for a new drug designed to prevent Clostridioides difficile infections.
In an article in The Lancet Infectious Diseases, researchers from Synthetic Biologics of Rockville, Maryland, report that the oral beta-lactamase enzyme ribaxamase, when given to patients with lower respiratory tract infections in conjunction with intravenous ceftriaxone, reduced the incidence of C difficile by 2.4 percentage points compared with placebo—a 71% relative risk reduction.
The authors of the study, which was funded, designed, and conducted by Synthetic Biologics (developer of ribaxamase), say the findings support continued clinical development of the drug.
Ribaxamase is designed to reduce C difficile risk by limiting the collateral damage caused by broad-spectrum beta-lactam antibiotics. Once in the gut, these antibiotics disrupt the natural balance of the gut microbiome and pave the way for opportunistic C difficile bacteria to spread. Ribaxamase works by degrading excess intravenous beta-lactam antibiotics that are excreted into the upper gastrointestinal tract, without interfering with the efficacy of the drugs.
Risk reduction, safety concerns
In the double-blind, multicenter randomized trial, patients older than 50 years with moderate-to-severe lower respiratory tract infections were recruited from 84 sites in the United States, Canada, Bulgaria, Hungary, Poland, and Serbia. After screening for inclusion in the study, the patients were randomly assigned 1:1 to receive either 150 milligrams of ribaxamase or placebo during ceftriaxone treatment, and for 72 hours after treatment.
The trial investigators chose ceftriaxone because, among beta-lactams, it is highly excreted in bile and confers a particularly high risk of C difficile infection. The trial also focused on older patients, because they tend to be more vulnerable to C difficile infections.
The primary end point of the study was a diagnosed C difficile infection in patients who had received at least one treatment dose, either during the treatment or for 4 weeks following treatment. Safety outcomes were also assessed.
A total of 413 patients were enrolled in the study, with 207 patients assigned to receive ceftriaxone plus ribaxamase and 206 assigned to the placebo group. One patient in the ribaxamase group withdrew consent, so the modified intention-to-treat population had 206 patients in each group. Overall, two patients in the ribaxamase group (1.0%) met the criteria for C difficile infection, compared with seven patients (3.4%) in the placebo group (risk reduction 2.4 percentage points; 95% confidence interval [CI], -0.6 to 5.9, P = 0.045).
The trial results also showed a significant reduction in the risk of colonization with vancomycin-resistant enterococci in the ribaxamase group at the end of treatment and at the end of the 4-week follow-up period, a finding the authors say indicates the drug's ability to protect the gut microbiome from other opportunistic pathogens. In addition, the lower respiratory tract infection was cleared in similar numbers of patients in the two groups, which suggests that ribaxamase did not reduce the effectiveness of the ceftriaxione.
The safety analysis found that 84 patients (40.8%) in the ribaxamase group and 91 (44.2%) in the placebo group reported adverse events, with most being mild or moderate. But serious adverse events were more numerous in the ribaxamase group than the placebo group (16% vs. 10.2%), as were deaths. Fatal adverse events were recorded in 11 patients (5.3%) who took ribaxamase, compared with 5 of the patients (2.4%) who took the placebo.
The investigators believe this imbalance in deaths is connected to the patients' medical history. They note that six of the patients who died in the ribaxamase group died of cardiac-associated causes, compared with one patient in the placebo arm, and a post-hoc analysis found that the ribaxamase group had more than twice the number of patients with pre-existing cardiac-associated disorders than did the placebo group (23 patients vs. 10). None of the deaths was considered related to the drug.
In April 2018, the US Food and Drug Administration (FDA) withdrew the breakthrough drug designation for ribaxamase because of the death imbalance in the trial. A planned phase 3 trial, which will evaluate efficacy of the drug in a broad patient population with a variety of infections treated by different beta-lactam antibiotics, is expected to address those concerns. The FDA gave Synthetic Biologics the go-ahead for the phase 3 trial in November 2018 and is currently reviewing the protocol for the trial.
Prevention before cure
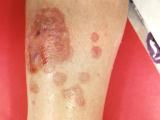
Depending on what happens in the phase 3 trial, ribaxamase could be the first drug on the market for the prevention of C difficile, a difficult-to-eradicate bacterium that has become one of the leading causes of hospital-acquired infections in the United States and is labeled an "urgent threat" by the Centers for Disease Control and Prevention. According to the most recent studies, C difficile causes more than 450,000 infections each year in US hospitals, is associated with nearly 30,000 deaths, and costs the US healthcare system more than $5 billion annually.
Drugs like fidaxomicin and bezlotoxumab, along with fecal microbiota transplantation, have shown promise in reducing recurrent C difficile infections, which occur in approximately 20% of patients. Cutting down on the incidence of recurrence would help reduce the overall burden of the disease.
But as infection control specialist Simon Goldenberg, MBBS, argues in a commentary that accompanies the study, finding a way to prevent C difficile infection in the first place, and lessen the gut microbiome disruption caused by many antibiotics, would get to the root of the problem.
Goldberg writes, "However, the old maxim that prevention is better than cure is particularly germane in this case. Protecting the gut microbiota from the deleterious effect of antimicrobials initially, rather than seeking to restore it after the damage has been done, seems to be the most effective strategy."
Other potential options for preventing C difficile include vaccines. Pfizer is currently conducting a phase 3 trial for PF-06425090, a vaccine that works by neutralizing the two main disease-causing toxins produced by C difficile.
See also:
Mar 15 Lancet Infect Dis study
Mar 15 Lancet Infect Dis commentary