Oct 23, 2012
Saudi health ministry says it was bypassed in reporting of novel coronavirus
Saudi Arabia's deputy minister of public health, Ziad A. Memish, MD, has complained publicly that the initial report of the recently discovered novel coronavirus in a Saudi patient bypassed internal reporting channels in Saudi Arabia. Memish posted his statement yesterday on ProMED, the online reporting service of the International Society for Infectious Diseases, the same service that published the initial report of the novel virus, by Saudi physician Ali Mohamed Zaki, MD, PhD, on Sep 20. Memish also complained that his office was not contacted by the Toronto Star, which reported on Oct 21 that Zaki said the Saudi health ministry was angry with him and had forced the hospital where he worked to fire him. Memish wrote, "Unfortunately, my ministry was not contacted by the Toronto Star nor by ProMED-mail's moderator to discuss pending reports of our novel coronavirus. ProMED-mail's readership has been left in the dark about internal reporting mechanisms that were either intentionally or inadvertently circumvented in this situation." He went on to note that Saudi Arabia bears an "enormous" public health responsibility for the millions of Hajj pilgrims visitng Mecca at this time of year. "We invite our friends and colleagues to stay tuned; we invite ProMED-mail to collaborate with us to balance public health reporting. As of now, the full story has yet to be told," he concluded. The novel virus was isolated from a 60-year-old Saudi man who died of a respiratory infection in a Jeddah hospital on Jun 24. A Qatari man infected with the same virus remains hospitalized in the United Kingdom.
Oct 22 ProMED post by Memish
Oct 21 Toronto Star story
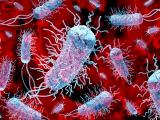
NC Fair E coli outbreak cases rise to 100
The number of people sickened in an Escherichia coli O157:H7 outbreak linked to attendance at a North Carolina county fair has risen to 100, 62 of them children, the North Carolina Department of Health and Human Services (NCDHHS) said today. An investigation is pointing to animal contact as the likely source, but officials still haven't pinpointed the exact cause, according to an Oct 19 NCDHHS statement. Authorities have interviewed patients and families, as well as 150 people who attended the fair but did not get sick. Laura Gerald, MD, MPH, North Carolina's state health director, said investigators may not be able to identify a single source but hope to have more conclusive results within a month. "Our goal throughout this investigation is to identify how to prevent similar outbreaks and deaths in the future," she said in the statement. Meanwhile, the Cleveland County Fair announced on Oct 19 that it was temporarily closing the fairgrounds to public events as a health precaution until health officials complete their investigations. The outbreak total rose by two cases since yesterday. Thirteen patients have been hospitalized, and cases includes one fatality, in a 2-year-old boy.
Oct 23 NCDHHS E coli case count
Sanofi says quadrivalent flu vaccine performed well in phase 2 and 3 trials
Sanofi Pasteur's quadrivalent influenza vaccine (QIV) containing two influenza B strains compared well with standard trivalent flu vaccines (TIVs) in phase 2 and 3 clinical trials to assess immunogenicity and safety, the company announced yesterday. An overview of the results was presented at IDWeek in San Diego, a joint meeting of four infectious-disease societies. Last week Sanofi announced it had applied for licensing of Fluzone QIV, which is designed to address the difficulty of predicting which of two type B flu lineages, Victoria or Yamagata, will be more common each flu season. The company conducted a phase 2 trial involving 570 nonelderly adults, a phase 3 study in 675 elderly people (65 and older), and a phase 3 study involving 4,347 children aged 6 months through 8 years. Participants were randomly assigned to receive the QIV or one of two TIVs, each containing either a Victoria-lineage or a Yamagata-lineage B strain. All four flu strains in QIV were found to be non-inferior to the two TIV formulations in 35 of 36 analyses in adults, elderly people, and children, the company said. In elderly people, the QIV missed non-inferiority to TIV for influenza A/H1N1 on one measure of immunogenicity, but it achieved non-inferiority on two other measures. Further, in 15 of 16 analyses in children and the elderly, QIV induced statistically superior levels of antibodies (geometric mean titers) and seroconversion rates for each B strain in comparison with the TIV version that lacked the corresponding B strain. The safety profiles of the quadrivalent and trivalent vaccines were not materially different, Sanofi said.
Oct 22 Sanofi press release
Ebola antibodies found in Chinese bats
Bats in China have been found to harbor antibodies to Ebola viruses, the first such evidence of filovirus infection in the country, researchers reported in the Virology Journal. Chinese and Australian researchers developed an enzyme-linked immunosorbent assay (ELISA) for Ebola using the recombinant nucleocapsid protein and conducted a seroprevalence survey among Chinese bats. The researchers collected 843 wild bats from various provinces in mainland China from 2006 to 2009. They found that 32 of 843 serum samples contained Ebola antibodies and that 10 of 16 were further confirmed by western blot test. They conclude, "To our knowledge, this is the first report of any filovirus infection in China."
Oct 13 Virol J abstract