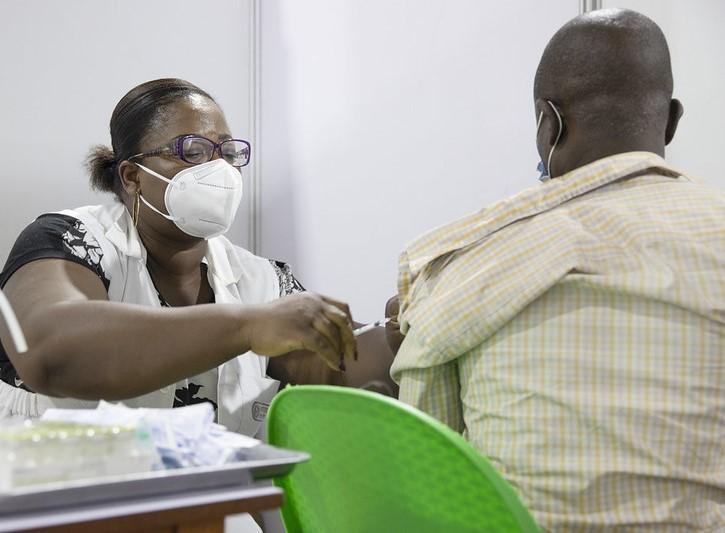
Moderna yesterday reported promising phase 3 clinical trial findings for its candidate combination mRNA vaccine (mRNA-1083) against influenza and COVID-19. The vaccine contains components of Moderna's candidate seasonal flu vaccine and its next-generation COVID-19 vaccine.
The company tested the vaccine in two adult cohorts, one adults ages 65 and older—some who were coadministered Fluzone high-dose flu vaccine and Moderna's current COVID vaccine and some who got Moderna's combination vaccine. The other group comprised adults ages 50 to 64 years old, some of whom were co-administered Fluarix, a standard-dose flu vaccine, and Moderna's current COVID vaccine and some who got Moderna's combo vaccine.
In both groups, the combo vaccine prompted higher immune response against seasonal flu strains and SARS-CoV-2. Safety and tolerability measures were similar to that of licensed vaccines used in the trial.
Moderna said it will present the findings at an upcoming conference and will submit them for publication in a medical journal. The company said it would engage with regulators on the next steps.
Combo vaccine could help fill vaccination gaps
In a blog post, Francesca Ceddia, MD, Moderna's chief medical affairs officer, said other combinations such as tetanus, diphtheria, and pertussis (TDaP) and measles, mumps, and rubella (MMR) have been shown to protect against multiple disease and have been tied to improved vaccine coverage and adherence to immunization schedules.
She said that, as of May, flu vaccine uptake for the 2023-24 season was more than double that of COVID for US adults. Ceddia added, however, that COVID hospitalizations were higher than for flu, especially in older adults. "The convenience that a combination vaccine could offer patients may help fill this gap while simplifying and routinizing vaccination against both diseases, which could help improve compliance with public health recommendations."