- The US government has announced that its offer of free at-home COVID tests will end on March 8. The Department of Health and Human Services (HHS) had announced another round of free tests by mail back in November 2023, heading into the winter respiratory virus season. HHS said tests in this latest round of free tests may show expired dates, but the Food and Drug Administration has extended the dates. HHS is still offering free COVID testing for uninsured people who were exposed or have symptoms through the Centers for Disease Control and Prevention Increasing Community Access to Testing program, which has about 10,000 sites.
- Flu activity remains elevated in Northern Hemisphere countries, though overall detections decreased at the global level, the World Health Organization said yesterday in its latest update, which covers roughly the first half of February. Some West Asian countries reported increases, including Georgia, Israel, and Oman. In Southeast Asia, increases were mainly driven by rises in Malaysia and Singapore. Of respiratory samples that were positive for flu at national labs, 72.4% were influenza A, and, of subtyped influenza A viruses, 54.2% were the H3N2 strain and 45.8% were 2009 H1N1.
- Three US states—Minnesota, New York, and Ohio—have reported more highly pathogenic avian flu outbreaks in poultry, according to the latest updates from the US Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS). All involved backyard flocks. The pace of outbreaks has declined steeply since December, but detections continue at a low level in poultry, with detections involving the H5N1 strain still frequently reported in wild birds.
Quick takes: No more free at-home COVID tests, high flu levels in Northern Hemisphere, avian flu in 3 states
Case report: 217 COVID vaccine doses haven't harmed man's immune system
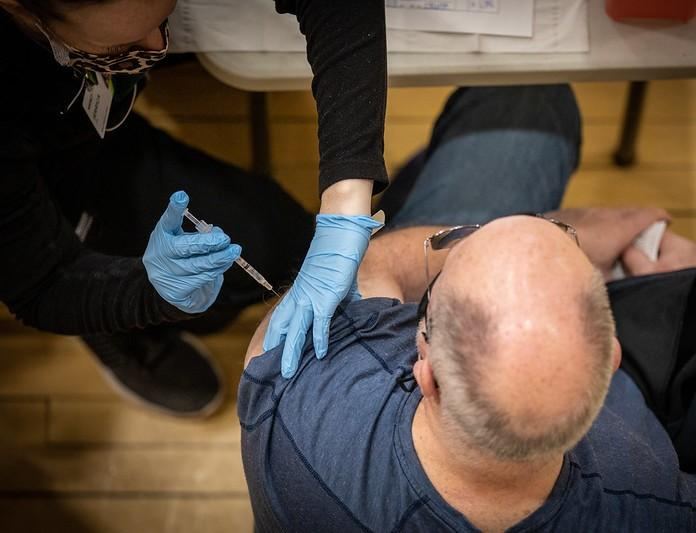
A German man who claims to have received 217 doses of eight different COVID-19 vaccines for "private reasons" has a fully functional immune system and much higher concentrations of immune cells and antibodies against SARS-CoV-2 than those seen in recipients of three doses, researchers report in The Lancet Infectious Diseases.
Of the 217 doses the man said he received over 29 months, 134 have been officially confirmed.
The 62-year-old man from Magdeburg agreed to undergo medical testing in Erlangen after researchers there read newspaper articles on his case and were interested in learning the immunologic consequences of hypervaccination. He gave them access to the results of blood tests he had taken in recent years and donated blood samples for further testing as he continued to seek more doses of his own volition.
The public prosecutor in Magdeburg investigated the case for fraud but didn't file criminal charges.
Overvaccination not recommended
From November 2019 to October 2023, 62 routine clinical chemistry parameters revealed no abnormalities attributable to hypervaccination, and SARS-CoV-2 antigen tests, polymerase chain reaction (PCR) tests, and nucleocapsid serologic tests showed no evidence of past COVID-19 infection. The man reported no vaccine-related adverse effects.
The observation that no noticeable side effects were triggered in spite of this extraordinary hypervaccination indicates that the drugs [vaccines] have a good degree of tolerability.
Killian Schober, MD
The man had more T-effector cells against SARS-CoV-2 than did a control group of three-dose recipients, and these cells were just as effective in fighting the virus after long-term overexposure. He also had a similar number of memory T cells, the progenitors of effector cells. Indeed, his levels of SARS-CoV-2 antibodies increased significantly even after the 217th dose, and the immune system's effectiveness against other pathogens was unchanged.
"The observation that no noticeable side effects were triggered in spite of this extraordinary hypervaccination indicates that the drugs [vaccines] have a good degree of tolerability," senior author Killian Schober, MD, said in a Friedrich-Alexander-Universitat Erlangen-Nurnberg news release.
But the researchers recommend against receiving more than the recommended number of doses. "Current research indicates that a three dose vaccination, coupled with regular top-up vaccines for vulnerable groups, remains the favored approach," Schober said.
Study finds no link between pre-op urine testing, reduced post-op infections

A retrospective cohort study found that preoperative urine culture testing was not associated with a lower risk of urinary tract infections (UTIs) or surgical-site infections (SSIs), researchers reported yesterday in JAMA Network Open.
Although guidelines recommend against preoperative urine culture testing before nonurologic surgery because it frequently results in unnecessary antibiotics for asymptomatic bacteriuria, previous studies have found that up to 25% of nonurologic surgical procedures are preceded by a screening urine culture, with rates even higher in certain surgical specialties and implant procedures. To assess the relationship between preoperative urine culture and postoperative UTI and SSI, researchers analyzed surgical procedures performed from 2017 through 2019 at any of 112 Veterans Affairs (VA) medical centers.
The cohort included patients who underwent major elective noncardiac, nonurologic operations. The researchers used inverse probability of treatment weighting (IPTW) to balance characteristics between those did and did not undergo a urine culture.
No reduction in UTIs or SSIs
A total of 250,389 VA enrollees who underwent 288,858 surgical procedures were included in the study; 88.9% of surgical procedures involved male patients, and 48.9% patients were 65 years or older. Before IPTW, patients with a preoperative urine culture were older, more likely to have a history of UTIs, and have more comorbidities. Baseline characteristics were well balanced among treatment groups after applying IPTW weights.
Preoperative urine culture was performed for 10.5% of surgical procedures. The IPTW analysis found that preoperative urine culture was not associated with SSI (adjusted odds ratio [AOR], 0.99; 95% confidence interval [CI], 0.90 to 1.10) or postoperative UTI (AOR, 1.18; 95% CI, 0.98 to 1.40). In analyses limited to orthopedic surgery and neurosurgery as a proxy for prosthetic implants, the adjusted risks for UTI and SSI were also not associated with preoperative urine culture.
The study authors say they hope the results will change the costly and potentially harmful practice of urine testing and antibiotic use prior to surgery.
"Despite data and guidelines, deimplementation of ingrained practices is challenging," they wrote. "The findings from this study can inform educational interventions and can be used for audit and feedback interventions to deimplement routine, unnecessary urine cultures and antibiotic treatment."
With new award, CARB-X hits the 100-project mark in battling antibiotic resistance
CARB-X today announced funding for its 100th project addressing antimicrobial resistance (AMR).
The $1.06 million award will help the Hemholtz Institute for Pharmaceutical Research Saarland (HIPS), of Saarbrucken, Germany, develop a new class of small-molecule inhibitors of bacterial sliding clamp, a pivotal component of DNA replication machinery that has not previously been targeted. The novel compounds have shown promising antibacterial activity against several pathogens that cause community-acquired bacterial pneumonia (CABP).
Lower respiratory tract infections, including CABP, are estimated to have killed 2.6 million people globally in 2019, more than 400,000 of whom died from infections caused by antibiotic-resistant bacteria.
"With this 100th project, we are doubling-down on our support of novel approaches to deliver antibiotics that clinicians and patients need," CARB-X (Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator) R&D chief Erin Duffy, PhD, said in a press release. "If successful, the HIPS project will offer a workhorse antibiotic for community-acquired infections that will also take the pressure off antibiotics in the [World Health Organization] model list of essential medicines."
Boosting a vulnerable pipeline
Since its founding in 2016, CARB-X has played a critical role in efforts to boost the pipeline for new antibacterials and other products targeting drug-resistant bacteria, awarding $452.6 million for early-stage development of vaccines, diagnostic, antibiotics, and other therapies. Eighteen of its 100 projects have made it into first-in-human trials, 12 remain in clinical development, and 2 diagnostics have reached the market.
"When CARB-X started in 2016, the antibacterial pre-clinical pipeline looked promising but vulnerable," said CARB-X Executive Director Kevin Outterson, JD. "Even the teams with the most impactful ideas lacked the capital and support needed to advance their R&D products towards patients. CARB-X stood in the gap, and, eight years later, we have clear proof that our model is working."
Study: Pooled flu vaccine protection hovers around 41%
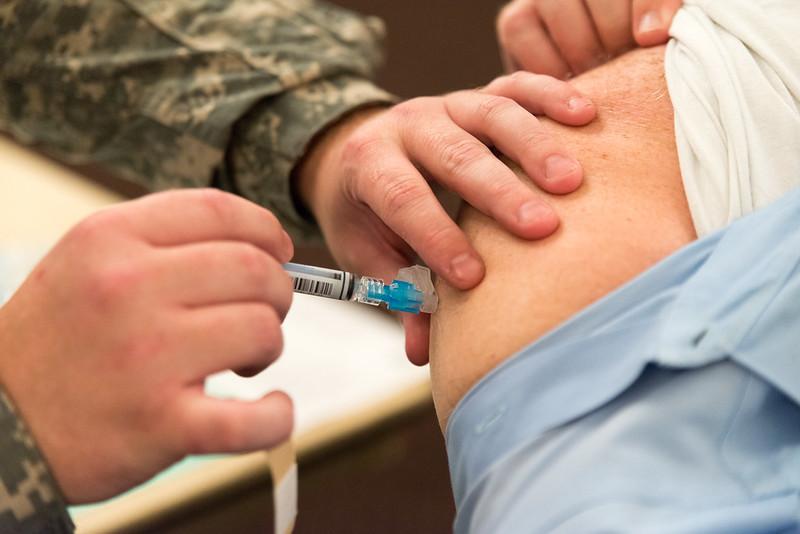
A new large meta-analysis of flu vaccine effectiveness (VE) studies conducted from 2017 through 2022 shows an average VE of 41.4% for the annual flu shot, with highest protection offered against the H1N1 strain and the lowest against the H3N2 strain. The study was published last week in Vaccine.
A total of 191 studies estimating seasonal VE for 1,221,809 participants were included in the final analysis.
In total, pooled VE was 41.4% (95% confidence interval [CI], 39.2% to 43.5%). VE was 55.4% against H1N1 (95 % CI, 52.7% to 58.1%) and 26.8% against H3N1 (95% CI, 23.5% to 29.9%).
For influenza B strains, VE was 47.2% (95% CI, 38.1% to 54.9 %) against the Yamagata lineage and 40.6% (95% CI, 23.7% to 53.7%) against the Victoria lineage, the authors said.
Younger children more protected
Overall pooled VE was 39.2% in preventing flu-associated outpatient visits and 43.7% in preventing flu-related hospitalizations.
An analysis that accounted for recipients' ages revealed that VE decreased with age across all studies. For children, pooled VE was 48.6% (95% CI, 44.7% to 52.2%). For adults aged 18 to 64 years, pooled VE was 36.7%.
Influenza vaccination provided moderate effectiveness against laboratory-confirmed influenza,
"Our study demonstrated influenza vaccination provided moderate effectiveness against laboratory-confirmed influenza, and induced comparable protection across the disease's clinical spectrum," the authors concluded.
In case you missed it
This week's top reads
Our underwriters
Unrestricted financial support provided by











