May 8, 2012
NIH awards $8.1 million for new drugs against Ebola, plague, Marburg
The National Institutes of Health has awarded the University of Washington (UW), the University of Texas, and Seattle-based Kineta Inc. an $8.1 million biodefense grant to develop new drugs to treat Ebola, plague, Marburg, yellow fever, and other diseases, according to a Kineta news release today. The funds will help Kineta move two small-molecule drug candidates closer to clinical trials. Its program, Agonists of the Retinoic Acid Inducible Gene I (RIG-I) Innate Immune Pathway, focuses on such high-impact viruses as influenza, hepatitis C, West Nile, and respiratory syncytial virus. RIG-I is a molecular "on/off" switch that triggers the body's innate immune system, the company said in the release. The grant will increase the number of disease targets to include less common henipaviruses and filoviruses, such as yellow fever, Ebola, Marburg, Japanese encephalitis, and plague. "These diseases are major concerns of the United States government for their risk of sparking a pandemic and their potential use as bioterrorist weapons. By utilizing an innate immune pathway we hope to develop better drugs that won't be outsmarted by viral mutation," UW professor Michael Gale, Jr., PhD, principal investigator of the grant, said in the news release.
May 8 Kineta news release
Controversy over beef product forces closing of three plants
South Dakota–based Beef Products Inc. (BPI) will permanently close three of its four plants that make "lean finely textured beef" (LFTB) because of attacks on the product, which critics call "pink slime," the Associated Press (AP) reported yesterday. BPI had suspended operations at the three plants, located in Amarillo, Tex., Garden City, Kan., and Waterloo, Iowa, when the controversy peaked in March. The closures will mean the loss of 650 jobs. A BPI plant in South Sioux City, Neb., will keep operating but at a lower rate. BPI blamed the closures on what it said were unfounded attacks on its product, which consists of beef trimmings that are heated slightly, centrifuged to remove liquefied fat, and treated with ammonia to kill bacteria. LFTB has been used for years in ground beef, including in the US Department of Agriculture's (USDA's) school lunch program, but was not listed on labels. No foodborne disease outbreaks have been linked to it, but recent consumer opposition caused some grocery and restaurant chains to drop it and prompted the USDA to allow schools to opt out of using it. The company had hoped to recover from the setback but has concluded that doing so would be impossible in the near term, BPI spokesman Rich Jochum told the AP. Marion Nestle, a nutrition and food studies professor at New York University, told the AP that BPI misinterpreted consumers' concern about LFTB as being focused on safety rather than on not knowing what was in their food.
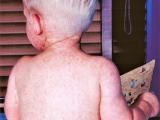
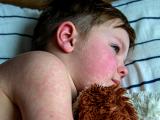
Measles activity off to a slower start in Europe
Measles activity in European countries is lower so far this year compared this point in the 2010 and 2011 seasons, the European Centre for Disease Prevention and Control (ECDC) said today in its latest update, which covers the first 2 months of the year. Through the end of February, countries reported 1,447 cases, compared with 5,731 during the first 2 months of 2011 and 5,752 during the same period of 2010. Fewer outbreaks have been reported in most countries, except for the United Kingdom, Romania, and Spain. Among several ongoing UK outbreaks, one in Merseyside is the largest in northwestern England since the measles, mumps, and rubella vaccine was introduced in 1988. Another hot spot is the Ukraine, which has reported 8,082 cases in six districts as of Apr 24. The ECDC said Romania was the only country to exceed 1 case per 100,000 population during the reporting period. Twelve countries reported no measles cases in January and February. Of 1,315 patients with known vaccination status, 83% were unvaccinated, and 75% of the vaccinated group had received only one dose of the measles vaccine.
May 8 ECDC statement