Oct 24, 2012 (CIDRAP News) – A federal panel today recommended that all pregnant women receive the tetanus, diphtheria, and pertussis (Tdap) vaccine during each pregnancy and that infants at increased risk receive four doses of a newly approved meningococcal combination vaccine.
The federal Advisory Committee for Immunization Practices (ACIP), whose recommendations are usually accepted by the Centers for Disease Control and Prevention (CDC), made the Tdap recommendation on a 14-0 vote (with one abstention) and the meningococcal shift on a 13-1 vote, also with one abstention.
The Tdap step expands the ACIP's June 2011 recommendation that, among pregnant women, only those who have not had the Tdap vaccine should receive it.
"By getting Tdap during pregnancy, maternal pertussis antibodies transfer to the newborn, likely providing protection against pertussis in early life, before the baby starts getting DTaP [the children's version of Tdap] vaccines," the CDC said in a press release e-mailed to reporters. "Tdap will also protect the mother at time of delivery, making her less likely to transmit pertussis to her infant."
The ACIP said that if women do not receive the vaccine during pregnancy, they should receive it immediately after giving birth, before they leave the hospital.
The CDC said the United States is on track to have the most reported cases of pertussis (whooping cough) since 1959, with more than 32,000 cases already reported, including 16 deaths, most of which were in infants.
The ACIP also approved a vaccine against Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y—called the HibMenCY vaccine—for the first time for at-risk infants. The committee said infants at increased risk for meningococcal disease should be vaccinated at 2, 4, and 6 months, and again at 12 through 15 months.
In a press release via e-mail, the CDC said those at increased risk included "infants with recognized persistent complement pathway deficiencies and infants who have anatomic or functional asplenia including sickle cell disease." The agency added, "HibMenCY can be used in infants ages 2 through 18 months who are in communities with serogroup C and Y meningococcal disease outbreaks."
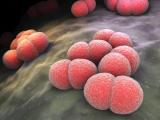
The two most common illnesses caused by N meningitidis are meningitis and bloodstream infections, the CDC said, adding that about 50 cases of meningococcal disease occur each year in infants and may be preventable.
"The majority of infant cases are caused by a type of the bacteria that are not prevented by meningococcal vaccines," the CDC said, referring to the two available vaccines given to people 11 years old and older.
H influenzae can also cause meningitis in children.
HibMenCY was approved by the US Food and Drug Administration in June for infants 6 weeks to 18 months old, according to the CDC Web site. It is made by GlaxoSmithKline. Sanofi's meningococcal vaccine, MenACWY-D, or Menactra, is approved by the FDA for babies aged 9 to 23 months but does not contain the Hib component.
ACIP recommendations become official when they are published in the CDC's Morbidity and Mortality Weekly Report.