The US Center for Disease Control and Prevention (CDC) finalized its recommendation for Princeton University students and some staff to receive an imported vaccine against meningococcal serogroup B disease in the wake of an outbreak involving eight confirmed cases since March.
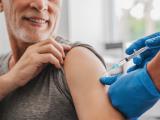
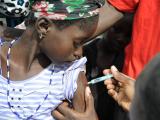
University health officials will offer the vaccine Dec 9 through 12, with a second dose needed for maximum protection offered in February, the school said yesterday in a statement.
Vaccine doses will be limited to at-risk groups covered in the CDC's recommendation: undergraduate students, graduate students living on campus, and members of the university community with certain underlying medical conditions, including those with spleen conditions and complement pathway disorder.
Princeton is covering the cost of the vaccine, which the Food and Drug Administration (FDA) approved for import to use in the outbreak under an Investigational New Drug application.
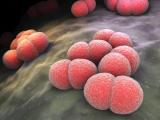
The vaccine against Neisseria meningitidis serogroup B, called Bexsero, is made by Novartis. Drug regulators in the European Union approved the vaccine in January, and Australian officials cleared it in August. In background materials, Novartis said dominant groups of meningococcal disease vary by country and region and can change over time, making it an unpredictable public health threat.
In the United States, serogroup B accounts for about a third of all meningococcal disease infections. However, in Australia serogroup B causes about 85% of meningococcal disease and sepsis cases, a percentage that has risen in recent years as illnesses linked to other serogroups have fallen, Novartis said in an Aug 15 statement on approval of the vaccine in Australia.
Princeton is one two US college campuses reporting a meningitis outbreak linked to serogroup B. Three students at the University of California at Santa Barbara (UCSB) have been diagnosed as having infections involving the same serogroup. County health officials, the school, and the CDC have had preliminary discussions about the vaccine there.
Many colleges require the meningococcal vaccine for their students, given that the disease is spread by respiratory secretions during close or lengthy contact, such as in dormitory settings. However the vaccine approved in the United States covers only four serogroups: A, C, Y, and W-135.
The CDC has said though sporadic cases aren't surprising, it is unusual to have two college outbreaks at the same time on two different coasts. It also said the outbreaks aren't related to each other—the strains have different genetic fingerprints.
In its most recent update on the outbreaks and the use of the vaccine, the CDC said Novartis has completed phase 2 clinical trials in the United States for its serogroup B vaccine. After reviewing licensing requirements, current vaccination schedules, and input from public health experts, the company decided to pursue advanced development of a meningococcal vaccine that covers all five serogroups.
The CDC said it and the FDA will work with Princeton University to monitor the safety of the serogroup B vaccine when immunization begins.
See also:
Nov 26 Princeton University press release
CDC Q and A on the vaccine (updated Nov 27)
Nov 25 CIDRAP News story "CDC urges vigilance over college meningitis outbreaks"
Aug 15 Novartis press release
Novartis meningococcal disease fact sheet