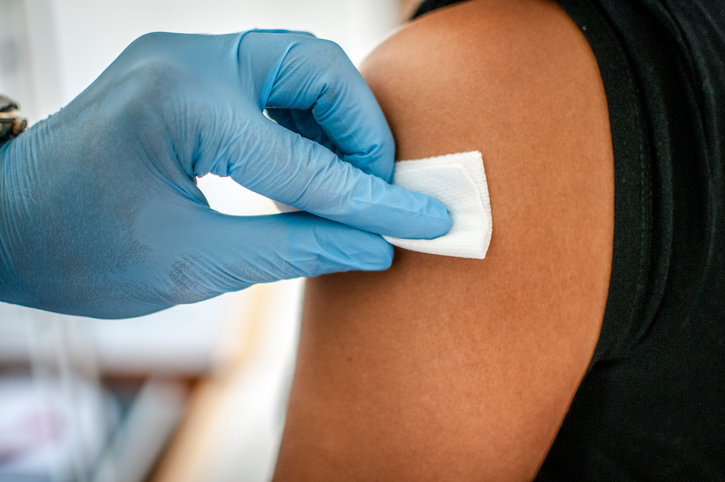
A survey of US pediatricians and family practitioners (FPs) revealed significant gaps in their knowledge about serogroup B meningococcal (MenB) vaccines and in their advocacy of the vaccines for young patients, according to a report yesterday in Pediatrics.
Two MenB vaccines are licensed in the United States, and in 2015 the Advisory Committee on Immunization Practices issued a “Category B” recommendation for use of the vaccines in 16- to 23-year-olds, the report notes. The recommendation means the vaccines "may be administered," but that clinicians should weigh the decision individually for each patient.
The survey targeted a nationally representative sample of 900 pediatricians and FPs who were contacted electronically between October and December of 2016. The survey drew a 72% response rate.
About half (51%) of the pediatricians and 31% of the FPs reported that they always or often discussed MenB vaccine with patients in the target age-group, the report says. Among those who usually discussed it, 91% recommended vaccination, compared with only 11% of those who rarely or never discussed the vaccine.
Many of the physicians "reported not knowing about factors influencing recommendation decisions," the authors said. For those who knew more, factors that increased the likelihood of recommending the vaccines included awareness of MenB disease outbreaks, disease incidence, and vaccine effectiveness, whereas awareness of the Category B (rather than Category A) recommendation decreased the likelihood.
The authors concluded that gaps in clinicians' knowledge of Men B disease vaccines appear to be "a major driver of the decision not to discuss the vaccines."
Need for clearer guidance
In an accompanying commentary, Michael T. Brady, MD, of Nationwide Children’s Hospital and the Ohio State University in Columbus, suggests that clinicians need clearer guidance on what to tell patients about MenB vaccines.
“Recommendations that require clinical decision-making need to provide clear guidance that informs providers so that they can determine what needs to be discussed with their patients and families and determine how strongly to recommend the vaccine,” he writes.
"Without this guidance, providers will continue to be challenged with Category B or permissive recommendations as suggested in the survey."
See also:
Aug 20 Pediatrics report and commentary