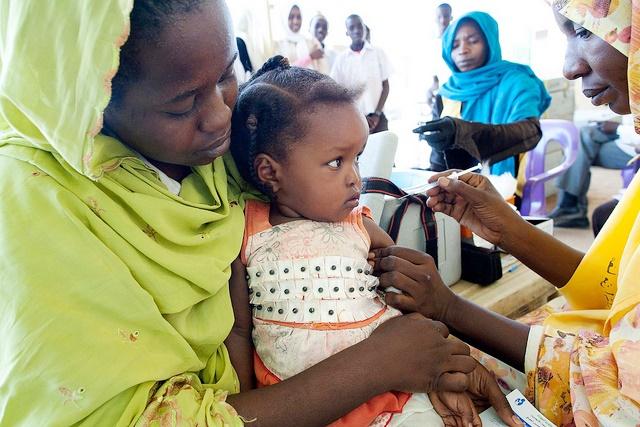
A vaccine that was quickly developed to curb a much-feared meningitis strain that affects only Africa has been a resounding success, but more steps are need to ensure that health programs in the affected countries ensure its long-term benefits, public health experts reported today.
Reports that document the impact of the vaccine, introduced to African countries in 2010, and those that sort out further clinical questions and policy issues, were published yesterday in a Clinical Infectious Diseases supplement supported by the Bill and Melinda Gates Foundation, the group that provided the $70 million in seed money in 2001 that got vaccine development started.
The supplement contains 29 articles, which include commentaries and cover topics that range from licensing and technical developments to lessons learned and clinical studies.
From 250,000 cases to 4
In 1996 an outbreak of meningitis, a strain found only in sub-Saharan Africa's "meningitis belt," sickened about 250,000 people and led to more than 25,000 deaths in just a few months. The epidemic prompted a plea for help from African health ministers, which led to the development of the vaccine (MenAfriVac), which costs just $0.50 a dose and is produce by the Serum Institute of India.
The vaccine that came from the global push was an improvement from older polysaccharide vaccines against the disease, which could be used only after epidemics were under way, couldn't be used to protect the youngest children, and offered only short-term protection.
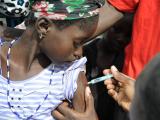
In a stunning turnaround, in 2013 the 26 countries in the meningitis belt reported only four lab-confirmed meningitis A cases. According to experts writing in the supplement, vaccine campaigns have reached more than 237 million people between ages 1 and 29 in 16 countries.
Cautions against complacency
Jean-Marie Okwo-Bele, MD, MPH, said in a WHO statement today that although the disease is almost eliminated in Africa, the job isn't yet done. "Our dramatic gains against meningitis A through mass vaccination campaigns will be jeopardized unless countries maintain a high level of protection by incorporating the meningitis A vaccine into their routine childhood immunization schedules," she said.
Ten countries still need to fully roll out the vaccine, which needs to find a place in routine immunization schedules, not just in campaigns.
A modeling study in the supplement suggests that if immunization programs aren't implemented after large campaigns take place, countries can expect to see resurgence in the disease after about 15 years.
Steve Davis, JD, president and chief executive officer of PATH, a nonprofit global health group in Seattle that helped develop the vaccine, said in the WHO statement, "The global community should not risk squandering this amazing lifesaving investment."
Long-lasting immune response
Among other new findings about the vaccine and its impact, studies suggest that 90% of people who received the vaccine still have antibodies 5 years later, which they said bodes well for even longer-term protection, which researchers will continue to track.
A study in the supplement also identified an added benefit of the vaccine: a protective immune response against tetanus. One of the reports found that tetanus levels in babies have dropped 25% in countries that have completed meningitis vaccine campaigns.
See also:
Nov 9 Clin Infect Dis supplement
Nov 9 WHO statement
Nov 9 Burness Communications press release