The Virginia Department of Health (VDH) recently reported a statewide meningococcal disease outbreak that has sickened 27 people since June 2022, including five patients who died from complications of their infections.
The illnesses involve Neisseria meningitidis serogroup Y, one of the strains included in the meningococcal conjugate vaccine (MenACWY). In its statement, the health department said the case total so far is three times higher than expected during the period. Twenty of the cases were reported in the eastern region of the state, but infections have also been reported in the southwest (5) and central (2) regions.
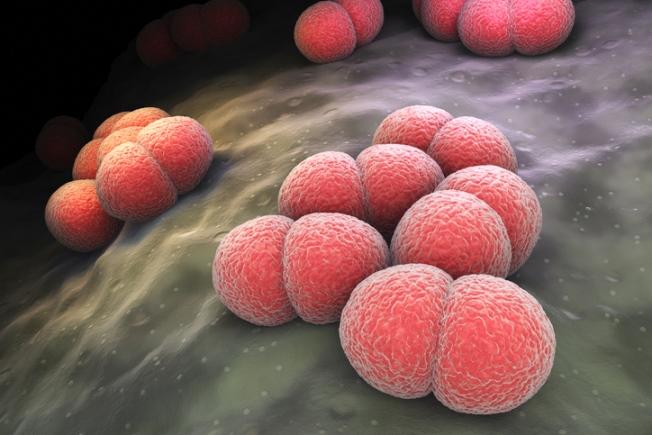
Impact on minority populations
So far, health officials haven't identified a common risk factor, though genetic sequencing of the bacteria suggests the infections are highly related. Most patients are Black or African American adults ages 30 to 60. Only one had received the MenACWY vaccine.
The VDH urged parents and healthcare providers to ensure that children receive all recommended vaccines, including MenACWY, which is recommended for adolescents before entering 7th grade, with a booster dose before 12th grade. The vaccine is also recommended for people who are at increased risk from the disease.
Meningococcal disease is rare but serious and is spread by lengthy contact through the exchange of respiratory or throat secretions, such as kissing or sharing cups or water bottles. The VDH said the overall risk to the state's population is low.
Along with the Virginia outbreak, the Centers for Disease Control and Prevention (CDC ) is also tracking a meningococcal disease outbreak involving serogroup C in Florida—primarily in gay and bisexual men—that since 2021 has sickened at least 24 people, 6 of them fatally. About half of the patients are Hispanic men, and some of the patients are living with HIV.

 A new
A new











