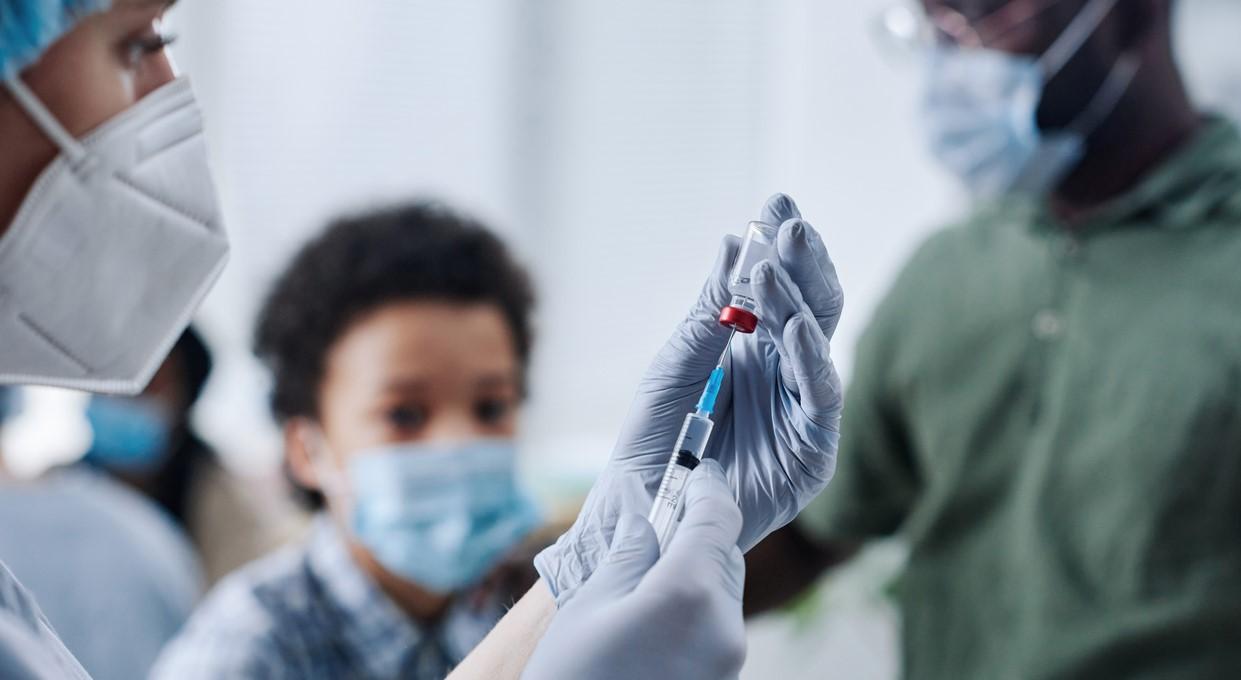
Bavarian Nordic and the Coalition for Epidemic Preparedness Innovations (CEPI) yesterday announced a partnership to advance the development of Bavarian Nordic's mpox vaccine for children in Africa.

CEPI has awarded $6.5 million to support phase 2 clinical trials of the MVA-BN nonreplicating vaccine in children ages 2 to 12 as compared to adults. The study will enroll 460 people in endemic regions who don't have a history of mpox illness or vaccination. Participants will receive two doses of the vaccine.
The study is expected to launch this year in one or more African countries.
Children hit especially hard
CEPI said the new partnership dovetails with a plan by African countries to coordinate efforts in to battle the virus, which was announced in April.
Richard Hatchett, MD, CEPI's chief executive officer, said children suffer disproportionately from mpox, a concerning and neglected disease that has spread rapidly in recent years.
Multiple countries in East and Central Africa are battling mpox outbreaks, and so far this year the Democratic Republic of the Congo has reported more than 6,500 cases, 345 of them fatal, with children accounting for most infections and deaths.