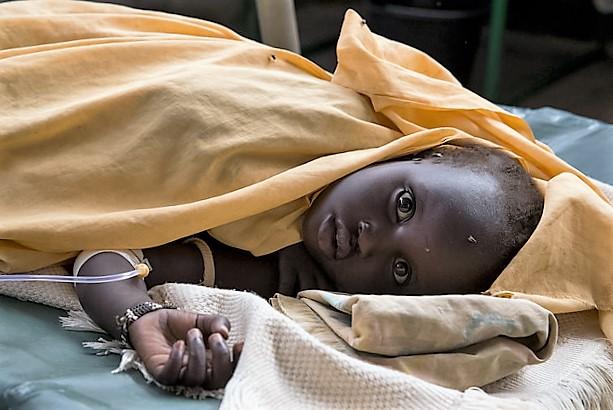
The United Kingdom today reported its first imported clade 1b mpox case, a patient who recently traveled to countries in Africa that are experiencing infection in community settings.
In a statement, the UK’s Health Security Agency (HSA) said the case was detected in London and that the patient has been transferred to the Royal Free Hospital’s High Consequence Infectious Diseases unit.
Susan Hopkins, MB BCh, chief medical advisor at the HSA, said, “The risk to the UK population remains low, and we are working rapidly to trace close contacts and reduce the risk of any potential spread. In accordance with established protocols, investigations are underway to learn how the individual acquired the infection and to assess whether there are any further associated cases.”
The UK is the fifth country to report an imported case of clade 1b monkeypox, alongside Sweden, Thailand, Germany, and India.
Different than clade 2 strain spreading globally, the novel clade 1b virus was detected amid a large outbreak in the Democratic Republic of the Congo (DRC) in April and is circulating alongside other clades and in Uganda.
Bavarian Nordic launches mpox trial in children, WHO adds more tests to EUL list
In other mpox developments, Bavarian Nordic announced yesterday that the first patients have been vaccinated in a clinical trial to assess the safety and immunogenicity of the Jynneos vaccine in children ages 2 to 11 years old. The trial is expected to enroll 460 participants, mainly in the DRC but with some sites to be included from Uganda.
Meanwhile, the World Health Organization (WHO) said today that it has added two more mpox PCR tests to its emergency use listing. They include Xpert Mpox from Cephid, which can be used on GeneXpert systems and deliver a result in 40 minutes. The other is the cobas MPXV assay, developed by Roche for use on cobas 6800/8800 systems. The test can detect both mpox clades and deliver a result in less than 2 hours.