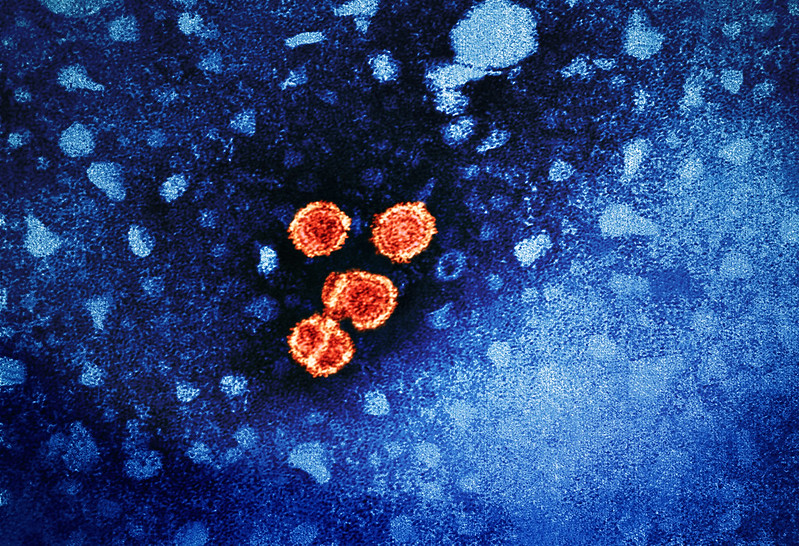
Pfizer today announced promising phase 3 clinical trial findings for a single dose of its respiratory syncytial virus (RSV) vaccine, Abrysvo, in adults ages 18 to 59 who are at increased risk of RSV infection and signaled that it would submit data to regulatory agencies as part of application for approval in that age-group.
In May 2023, the Food and Drug Administration (FDA) approved Abrysvo for use in adults ages 60 and older, and the Centers for Disease Control and Prevention (CDC) recommended its use in that age-group, based on shared decision making with health providers. In September 2023, the CDC recommended the vaccine for pregnant women as part of a strategy to protect vulnerable newborns.
Good safety, immune response
Pfizer said the new phase 3 findings are from a trial that examined safety and immunogenicity on adults ages 18 to 59 who are at risk for severe RSV infection such as those with asthma and diabetes. Currently, no RSV vaccines have been approved for this age-group.
Neutralizing responses against RSV-A and RSV-B were noninferior to those earlier trials in adults ages 60 and older. The younger adults had at least a fourfold increase in serum neutralizing titers 1 month following vaccination. The vaccine was well tolerated and had safety findings similar to trials in other populations.
Pfizer said it will publish the findings in a peer-reviewed medical journal and present them at an upcoming medical conference.