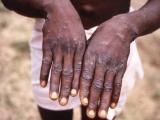
Sep 20, 2002 (CIDRAP News) – The smallpox vaccine being produced by Acambis plc for the US government worked well in its second trial, generating a characteristic vaccinia lesion in all recipients and causing no serious adverse events, the company said this week.
Earlier this month the British-based company announced successful results in the first phase I trial of the new vaccine, called ACAM1000. In that test, 30 people were given the new vaccine, called ACAM1000, and 30 were given Dryvax, the decades-old vaccine in the existing US stockpile. In the second phase I trial, 70 people were inoculated with ACAM1000 to gather more data on its safety and effectiveness.
The second trial "reinforced the results seen in the first Phase I trial, with 100% of subjects developing a 'take' within 10 days after vaccination and no serious adverse events," the company said in a news release. "In total, therefore, 100 previously unvaccinated subjects have received ACAM1000 and all of them experienced a vaccine 'take.'" The announcement was part of a report on the company's financial results for the first half of this year.
Acambis has begun a phase II trial in which three different doses of ACAM1000 will be used and compared with Dryvax, the company said. The firm has two contracts with the Department of Health and Human Services (HHS) to make a total of 209 million doses of smallpox vaccine, part of an effort to provide enough vaccine for all Americans by the end of this year. The company is working in partnership with US-based Baxter International.
Citing national security considerations, Acambis officials said they could not disclose how much vaccine they have produced so far, but added, "The first doses of final, filled and kitted smallpox vaccine have been produced for the [US] stockpile and the [Centers for Disease Control and Prevention] is fully supportive of both programmes and of the progress made to date."
According to a Washington Post report, Gordon Cameron, Acambis's chief financial officer, said the company may be in a position to bill HHS for large amounts of vaccine in December. Under government rules, that does not normally happen until a product is delivered and formally accepted, the report said.
The company announced it will soon start a phase I trial of ACAM2000 smallpox vaccine, which is being produced under the second contract with HHS. Both ACAM1000 and ACAM2000 are grown in cell culture rather than on the skin of calves, the method used to produce nearly all smallpox vaccine in the past.