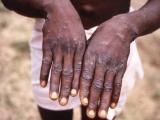
Mar 17, 2004 (CIDRAP News) Nine out of 250 people who received their first smallpox shots in a recent study reacted with a variety of self-limiting skin rashes, a side effect that could be mistaken for a serious reaction to the vaccine, according to a report in Clinical Infectious Diseases.
The rashes appeared on many parts of the body and in some cases caused itching or tingling but were not associated with other major symptoms, says the report by Richard N. Greenberg and colleagues at the Department of Veterans Affairs Hospital and University of Kentucky Medical School in Lexington, Ky.
The skin reactions were seen in a study comparing the standard smallpox vaccine, Dryvax, with a new vaccine produced in cell culture. (Both vaccines use vaccinia virus, but the cell-culture vaccine is expected to be safer because it is produced under more controlled conditions.) The study included 350 people, 100 of whom had had previous smallpox shots and the rest had not. Dryvax was administered to 100 volunteers, while 250 received the cell-cultured vaccine.
Benign rashes were seen in nine (3.6%) of the 250 first-time vaccinees versus none of the previously vaccinated volunteers. Of the nine, seven had received the cell-cultured vaccine and two had received Dryvax. There were no serious complications in any of the volunteers.
Five of the volunteers had urticarial rashes, and the other cases were classified as an exanthem, folliculitis, contact dermatitis, and erythematous papules. In six cases the rash was widespread on the body, but three volunteers had the rash only on their hands or their hands and feet. The rashes appeared an average of 13 days after vaccination and lasted an average of 11 days. Most of the volunteers took antihistamines and anti-inflammatories to control symptoms.
Greenberg, in a news release from the Infectious Diseases Society of America (IDSA), publisher of Clinical Infectious Diseases, said physicians should know that smallpox vaccine can cause benign rashes. A physician who was unaware of the possibility might wrongly assume that a rash was one of the more dangerous vaccinia complications, such as progessive vaccinia or eczema vaccinatum, he said.
"The concern is that the physician would overreact," Greenberg said. "These skin reactions are rather dramatic, and if we're going to be vaccinating against smallpox, the physician should know that these are benign complications and not subject the patient to unnecessary worry."
The report says that some of rashes could have been mild cases of generalized vaccinia, which occurs when the virus spreads from the vaccination site via the bloodstream and causes skin lesions elsewhere. "None of the rashes described in our volunteers resembled the primary pock lesion," the report states. "However, no diagnostic studies were conducted as part of this phase 1 trial. Additional studies will be needed to define the cause of these rashes."
The article notes that vaccinia-associated rashes have been reported frequently in the literature, but pictures and descriptions of the range of presentations have been relatively scarce. A study published in 2002, for example, noted rashes in 14.3% of 680 adults who received Dryvax shots.
Greenberg RN, Schosser RH, Plummer EA, et al. Urticaria, exanthems, and other benign dermatologic reactions to smallpox vaccination in adults. Clin Infect Dis 2004 Apr 1;38(7):958 [Full text]
See also:
Centers for Disease Control and Prevention: Smallpox Vaccine and Adverse Reactions: Guidance for Clinicians