Mar 6, 2003 (CIDRAP News) Two women recently contracted vaccinia infections of the eye as a result of contact with military personnel who had been vaccinated against smallpox, the Centers for Disease Control and Prevention (CDC) reported today.
Both patients were recovering after treatment with antiviral eye drops and, in one case, vaccinia immune globulin (VIG), the CDC reported in the Mar 7 issue of Morbidity and Mortality Weekly Report. This was the first reported case of treatment with VIG in the current civilian and military smallpox immunization programs.
The CDC also reported three other "serious" adverse events in smallpox vaccinees, including headache and dizziness, gallbladder inflammation, and hypertension. It was not known whether the vaccine was to blame for any of these.
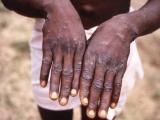
One of the ocular vaccinia cases involved a 26-year-old woman who shared a bed with a military vaccinee several times over a 3-week period after he was vaccinated, according to the report. The vaccinee often left his vaccination site uncovered. The woman suffered swelling, pain, and a discharge in the right eye, progressing to facial swelling and impaired vision. She was hospitalized Feb 22 and diagnosed with preseptal cellulitis without infection of the ocular globe.
After treatment with intravenous antibiotics, her condition improved and she was discharged, but she was readmitted the next day because of persistent blepharoconjunctivitis. Further tests showed the presence of vaccinia, and treatment with trifluridine eye drops and one dose of VIG led to improvement within 24 hours. The patient was discharged from the hospital Feb 28.
In the other case, an 18-year-old woman became ill after she handled the bandage of a military vaccinee, according to the report. She first had skin pustules 3 and 5 days after contact, and then had swelling in her right eye 8 days after contact. Initially she was treated with an oral antibiotic, but 2 weeks after contact she had two small lesions on her right eyelid, leading to the diagnosis of blepharoconjunctivitis. She was treated with trifluridine eye drops and improved noticeably within 24 hours.
The report said the 18-year-old woman had never had a smallpox shot, but it did not list the vaccination status of the 26-year-old.
In one of the other three serious adverse events, a woman suffered persistent headache and dizziness starting 5 days after her inoculation. She was admitted to a hospital, where neurologic tests showed no abnormalities, and was discharged with improved symptoms 2 days later.
The other two cases included a 46-year-old man who had severe hypertension and headache and a 51-year-old woman who suffered chest discomfort, shortness of breath, and nausea. The man was hospitalized for a day and treated with antihypertensive medication; the woman was found to have a gallstone and underwent surgery. Cholecystitis and hypertension are not known to be associated with smallpox vaccination, the report notes.
The CDC also reported 21 new "nonserious" adverse events, bringing the total for the civilian vaccination program to 46. The symptoms included fever, pruritus, rash, and pain, all of which are common reactions to the vaccine, the report said.
The report says that vaccinees who don't work in healthcare should cover their vaccination site with a gauze bandage and keep it dry by using a waterproof bandage when bathing. In addition, vaccinees should wear a long-sleeved shirt, especially in situations involving close contact, and should practice good hand hygiene.
The CDC has reported a total of three "moderate to severe" adverse events since the civilian vaccination program began Jan 24. These include the two ocular vaccinia cases reported today and a suspected case of generalized vaccinia, reported last week. The Department of Defense has reported two cases of encephalitis, one of myocarditis, seven suspected cases of generalized vaccinia, and one eye infection among the tens of thousands of troops vaccinated since December.
CDC. Smallpox vaccine adverse events among civiliansUnited States, February 25-March 3, 2003. MMWR 2003;52(9):180-181,191 [Full text]