May 20, 2004 (CIDRAP News) An arm of the National Institutes of Health (NIH) has awarded more than $40 million worth of contracts for research to reduce the risk of eczema vaccinatum (EV), a rare but severe complication of smallpox vaccination.
The contracts will launch the Atopic Dermatitis and Vaccinia Network (ADVN), consisting of two groups of medical institutions and one private research firm, according to the National Institute of Allergy and Infectious Diseases (NIAID).
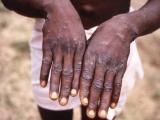
EV, which can be fatal, occurs almost exclusively in people who have a history of atopic dermatitis, the chronic skin condition also called eczema. EV can occur when eczema patients are exposed to vaccinia virus by receiving a smallpox shot or coming in contact with someone who has recently had the shot.
The primary aim of the research program is to provide information that will help in the design of a safer smallpox vaccine, the NIAID said in a news release yesterday.
"Previous studies suggest that both innate and adaptive immunity are impaired in patients with atopic dermatitis, but the specific defects that increase the likelihood of eczema vaccinatum have yet to be explained," said Daniel Rotrosen, MD, director of the NIAID's Division of Allergy, Immunology and Transplantation, in the news release. The research is expected to generate information that will "greatly influence the design of a safer smallpox vaccine."
The NIAID said the research network will consist of a Clinical Studies Consortium, an Animal Studies Consortium, and data coordinating center. The contracts cover a 5-year period, according to Petr Bocek, a medical officer in the NIAID Division of Allergy, Immunology and Transplantation. Bocek told CIDRAP News the funding includes about $20 million for the clinical studies group, about $11 million for animal studies, and about $10 million for data coordination.
The clinical studies group will investigate why people with eczema are susceptible to EV by studying their immune responses after natural exposure to less harmful skin viruses such as herpes simplex, the NIAID said. The institutions and principal investigators in the Clinical Studies Consortium are
- National Jewish Medical and Research Center, Denver; Donald Leung, MD, PhD
- Oregon Health and Science University, Portland; Jon Hanifin, MD
- Children's Hospital Boston; Lynda Schneider, MD
- University of California at San Diego; Richard Gallo, MD, PhD
- Johns Hopkins Asthma and Allergy Center, Baltimore; Lisa Beck, MD
- University of Bonn, Germany; Thomas Bieber, MD, PhD
The Animal Studies Consortium will establish animal models of atopic dermatitis and study their immune responses to vaccinia and other skin viruses. Institutions and principal investigators in the Animal Studies Consortium include
- Children's Hospital Boston; Raif Geha, MD, and Hans Oettgen, MD, PhD
- National Jewish Medical and Research Center; Donald Leung, MD, PhD, and Erwin Gelfand, MD
- Harvard Skin Disease Research Center, Boston; Thomas Kupper, MD, and Robert Fuhlbridge, MD
- University of Illinois at Chicago; Lawrence Chan, MD
- La Jolla (Calif.) Institute for Allergy and Immunology; Toshiaki Kawakami, MD, PhD
The Statistical and Data Coordinating Center will support the clinical and animal studies by analyzing research data, coordinating trials and regulatory activities, and developing a registry of atopic dermatitis patients, the NIAID reported. The center will be operated by Rho Federal Systems Inc. in Chapel Hill, N.C., with Susan Lieff, PhD, as the principal investigator.
Bocek said the research contracts were awarded through a competitive process in which groups of institutions submitted proposals, which were evaluated by NIAID committees.
Current estimates are that about 3% to 5% of the general population have eczema, according to Bocek. Historically, 10 to 39 cases of EV occurred per million people receiving their first smallpox shot, according to the Centers for Disease Control and Prevention (CDC). The NIAID statement said eczema vaccinatum can be fatal in 1% to 6% of cases, and the death rate may range up to 30% in children under 2 years old.
In the US military smallpox vaccination program, no cases of EV have occurred among the more than 623,000 people vaccinated since December 2002, according to the latest Department of Defense safety report. The CDC reported no EV cases among the 39,213 civilian healthcare workers who received smallpox shots in 2003. Bocek noted that the criteria for excluding people from smallpox vaccination are much stricter today than in the past.
See also:
May 19 NIAID news release
http://www.niaid.nih.gov/news/newsreleases/2004/Pages/advn.aspx
CDC fact sheet on adverse reactions to smallpox vaccine
http://www.bt.cdc.gov/agent/smallpox/vaccination/reactions-vacc-clinic.asp
Current safety summary of US military smallpox vaccination program
http://www.smallpox.mil/event/SPSafetySum.asp