Mar 13, 2003 (CIDRAP News) – Two young adults acquired vaccinia virus skin infections as a result of contact with military personnel who had received smallpox shots, and a third adult had a similar suspected vaccinia infection, according to the latest report of adverse events in the smallpox vaccination program.
The three cases bring the number of "moderate to severe" adverse events among civilians to six, the Centers for Disease Control and Prevention (CDC) reports in the Mar 14 issue of Morbidity and Mortality Weekly Report. The report says16,919 people had been vaccinated as of Mar 7, about 6 weeks after the civilian vaccination program started Jan 24. The CDC previously reported two probable cases of ocular vaccinia and a suspected case of generalized vaccinia.
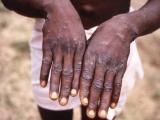
None of the three patients in the new cases had had a smallpox shot. One case involved a 23-year-old man who wrestled with a military recruit who had been vaccinated and had no covering over his inoculation lesion, the CDC reported. The man eventually experienced lesions on his chest, right shoulder, and face, along with mild malaise and right axillary lymhadenopathy. A polymerase chain reaction (PCR) test for vaccinia was positive.
In a second case, an 18-year-old woman sought care for a pustular lesion on her right forearm that had developed after close contact with her partner, a military member who had been vaccinated. The partner had kept the vaccination site covered with a small bandage but reported some oozing through the bandage. A specimen from the woman's lesion tested positive for vaccinia by PCR.
In the third instance, a 25-year-old woman went to an emergency department Mar 5 with three vesicular lesions on her right arm, according to the article. She reported having had close contact with a military vaccinee in mid-February. Results of tests for vaccinia and other viruses were pending when the report was written.
The CDC also reported four serious adverse events that occurred within a few days after smallpox vaccination but were types not known to be associated with the vaccine. These included chest pain and shortness of breath, diagnosed as angina, in a 43-year-old woman; vomiting and diarrhea in a53-year-old woman; exacerbation of chronic obstructive pulmonary disease, along with diarrhea and dehydration, in a 57-year-old woman; and viral myocarditis, thought to be associated with a previous influenza-like illness, in a45-year-old woman. All the patients were hospitalized briefly.
Officials also reported 30 new "nonserious" adverse events, including rash, fever, pruritus, and pain, which are not unusual following smallpox vaccination. A total of 76 nonserious adverse events have been reported in the civilian smallpox vaccination program so far.
In related news, CDC Director Dr. Julie Gerberding, and Dr. D. A. Henderson, leader of the global smallpox eradication program, received smallpox shots in front of reporters in Atlanta yesterday. The public vaccinations were part of the CDC's effort to spur the vaccination program for front-line healthcare workers, which has been slowed by workers' concerns about adverse reactions to the shots and compensation for resulting costs.
Plans call for vaccinating up to 450,000 public health and hospital workers who would serve on smallpox response teams in case of an outbreak. Federal health officials originally hoped this could be done within as little as a month from when the program started in January, but the 16,000shots given so far amount to a small fraction of that.
CDC. Smallpox vaccine adverse events among civilians—United States, March 4-10, 2003. MMWR2003;52(10):201-3 [Full text]