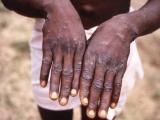
Dec 14, 2005 (CIDRAP News) – Exactly 100 of about 38,000 civilians who received smallpox shots in a federal program in 2003 suffered serious adverse events afterward, signaling that the program successfully screened out most people at risk for complications, according to a recent report.
The safety monitoring system "achieved its goal of safe administration of smallpox vaccine among a limited number of DHHS [Department of Health and Human Services] volunteers through successful exclusion of at-risk individuals and rapid detection of unexpected adverse events," says the report, published in the Dec 7 Journal of the American Medical Association.
The authors, with the Centers for Disease Control and Prevention (CDC), analyzed adverse events reported as a result of the smallpox vaccination program from January through October of 2003. HHS launched the program for healthcare and emergency response workers out of concern about the possibility of a terrorist release of smallpox virus. The report's first author is Christine G. Casey, MD, of the CDC's National Immunization Program.
Authorities originally hoped to vaccinate as many as 500,000 health workers, but only 37,901 received shots by the end of October 2003. Hospital and public health workers constituted 95% of those, with law enforcement and firefighters making up most of the rest, the report says. Most of the vaccinees (64%) were women, and more than 75% of them were between 40 and 64 years old and had received a smallpox shot before.
The Vaccine Adverse Event Reporting System (VAERS) received 822 adverse-event reports related to the vaccinations, the report says. Of these, 100 were classified as serious events, for a rate of 26.4 per 10,000 vaccinees.
Of the 100 serious events, 85 involved hospitalization or prolongation of hospitalization. Two people suffered permanent disability, and 10 experienced a life-threatening illness. The serious events included 21 classified as myopericarditis and 10 classified as ischemic events that were not expected on the basis of patient histories. Those 10 included six myocardial infarctions, two of which were fatal, and four cases of new or increased angina. Two cases of dilated cardiomyopathy occurring 2 to 3 months after vaccination were also reported.
As a result of cardiac adverse events in both civilian and military smallpox vaccinees, the CDC issued a Health Alert Notice on Mar 26, 2003, that described the events and recommended deferring vaccination for at-risk people. None of the 10 ischemic cardiac events in vaccinees occurred after the alert notice triggered cardiac screening of potential vacccinees, the report says.
The authors note that US military personnel recently vaccinated against smallpox have had a significantly increased rate of myocarditis, compared with unvaccinated military members. However, the report says the rate of ischemic cardiac events in civilian vaccinees does not appear to exceed the rate in a comparable unvaccinated population.
"Whether smallpox vaccination is causally associated with ischemic events remains uncertain," the authors write.
Two cases of generalized vaccinia and one case of postvaccinial encephalitis were reported in the program. But there were no cases of transmission of vaccinia (the vaccine virus) to others and no severe reactions requiring treatment with vaccinia immune globulin.
Among the 722 "nonserious" adverse events reported, the most common signs and symptoms were fever, 18.9%; rash, 18.4%; pain, 16.0%; headache, 15.2%; fatigue, 13.5%; and pruritus, 13.4%. Compared with those reporting nonserious events, people reporting serious events were more likely to be older than 40 (81% versus 64%).
People who had been vaccinated previously were slightly overrepresented among the vaccinees with serious adverse events, the authors found. They say this is not surprising, since the revaccinees were older than the primary vaccinees and may have had a higher risk of adverse events because of age-related underlying chronic disease.
The rates of expected, preventable, and noncardiac adverse events in the civilian vaccinees were about the same as rates in the much larger military vaccination program, the authors found.
Casey CG, Iskander JK, Roper MH, et al. Adverse events associated with smallpox vaccination in the United States, January-October 2003. JAMA 2005 Dec 7;294(21):2734-43 [Abstract]
See also:
Safety summary for Department of Defense smallpox vaccination program
http://www.smallpox.mil/event/SPSafetySum.asp