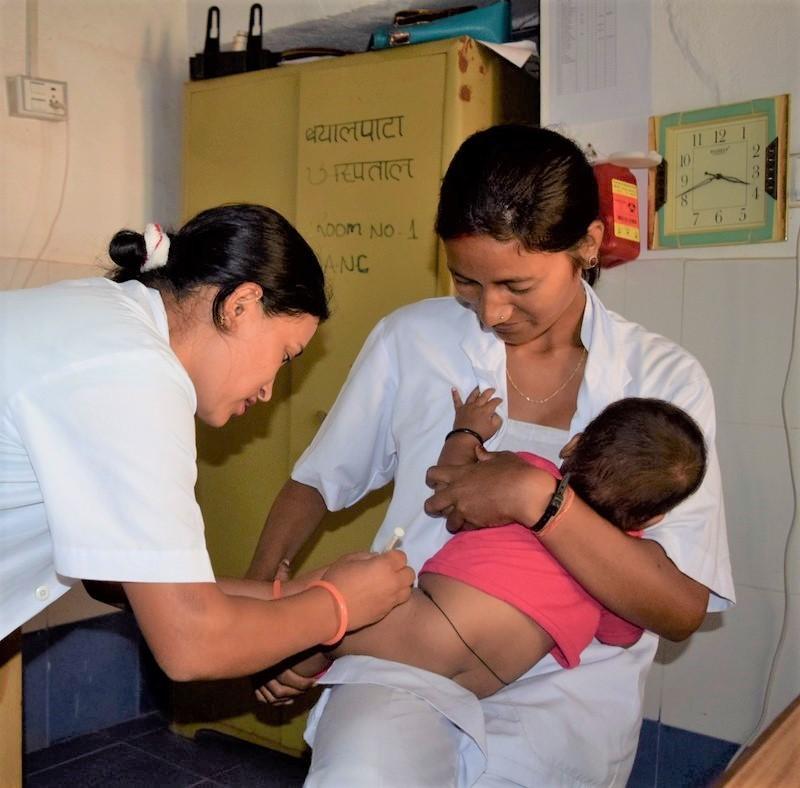
A single dose of a typhoid conjugate vaccine (TCV) was found to be immunogenic and effective in reducing typhoid fever in Nepalese children, according to interim results of a phase 3 trial published today in The New England Journal of Medicine.
To test the efficacy of the vaccine in an area where typhoid fever is endemic, investigators from the Typhoid Vaccine Acceleration Consortium (TyVAC) randomly assigned children 9 months to 16 years of age in Lalitpur, Nepal, to receive either a tetanus-toxoid conjugated Vi polysaccharide typhoid vaccine (Typbar-TCV) or a capsular group A meningococcal conjugate vaccine (MenA) as a control.
The primary outcome of the trial was typhoid fever—which is caused by Salmonella enterica serovar Typhi—confirmed by blood culture.
Vaccine efficacy of 82%
A total of 10,005 children received Typbar-TCV and 10,004 received the MenA vaccine. Analysis of the results showed that typhoid fever occurred in 7 children who received the TCV (79 cases per 100,000 person-years) and 38 who received the MenA vaccine (428 cases per 100,000 person-years). The protective efficacy of the TCV was thus 81.6% (95% confidence interval [CI], 58.8% to 91.8%; P < 0.001).
In the immunogenicity subgroup, seroconversion (a Vi immunoglobulin G level that at least quadrupled 28 days after vaccination) was 99% in the TCV group (677 of 683 children) and 2% in the MenA vaccine group (8 of 380).
The rate of adverse events in the first 7 days after vaccination was similar in both groups, with 5% of the TCV group and 5.4% of the MenA group reporting a fever, and 6.4% of the TCV group and 7.1% of the MenA group feeling generally unwell. A total of 132 serious adverse events occurred (61 in the TCV group and 71 in the MenA group), with 1 event being identified as vaccine-related.
"The efficacy of these results in an endemic population adds to a growing body of evidence supporting the use of TCV to reduce disease and save lives in populations that lack clean water and improved sanitation," Kathleen Neuzil, MD, MPH, director of the Center for Vaccine Development and Global Health at the University of Maryland School of Medicine and director of TyVAC, said in a press release from the University of Oxford.
TyVAC, which includes researchers from the University of Maryland School of Medicine, the Oxford Vaccine Group at the university, and PATH, an international nonprofit health group, was formed to aid countries in accelerating introduction of TCVs in developing countries. The organization is also conducting trials in Bangladesh, Burkina Faso, and Malawi.
Results confirm WHO recommendations
According to the most recent World Health Organization (WHO) estimates, between 11 million and 21 million typhoid cases occur each year worldwide, with at least 128,000 typhoid-related deaths. And in recent years, antibiotic-resistant strains of Salmonella Typhi have become increasingly common in South Asia, making the illness more difficult to treat.
Although testing of 41 blood-culture isolates from the confirmed typhoid fever cases in today's study found that most were non-susceptible to ciprofloxacin, no multidrug-resistant isolates were found, which is noteworthy given the current outbreak of extensively drug-resistant typhoid in neighboring Pakistan.
While the WHO has been recommending the use of vaccines against typhoid fever since 2008, to date no vaccines have been deemed suitable for widespread use in children in endemic areas. But in 2018, the WHO Strategic Advisory Group of Experts prequalified Typbar-TCV for use in children over the age of 9 months. That recommendation was made on the basis of a phase 3 trial and a human challenge study that showed that the vaccine had improved immunologic properties over other typhoid vaccines, was suitable for use in infants and young children, and provided longer-lasting protection.
Investigators will continue follow-up for 2 years to determine Typbar-TCV efficacy in the medium term and long term.
The trial was funded by the Bill & Melinda Gates Foundation.
See also:
Dec 5 N Engl J Med study
Dec 5 University of Oxford press release