The use of a rapid, biomarker-guided test to rule out ventilator-associated pneumonia did not reduce antibiotic use in intensive care unit (ICU) patients, researchers from the United Kingdom reported yesterday in The Lancet Respiratory Medicine.
The results come from a randomized controlled trial conducted in 24 ICUs in England, Scotland, and Northern Ireland. The trial was designed to determine whether measuring concentrations of the inflammatory biomarkers IL-1β and IL-8 in lung fluid could improve antibiotic stewardship in patients with clinically suspected ventilator-associated pneumonia (VAP).
Previous studies have validated low concentrations of IL-1β and IL-8 as effective markers for ruling out the illness and, which is frequently diagnosed in ICU patients and is a common reason for indiscriminate broad-spectrum antibiotic use. But the authors of the study say the findings don't necessarily mean that the test didn't perform well. Rather, they argue, the results reflect clinician skepticism about the diagnostic value of the test and entrenched beliefs about antibiotics.
Little effect seen in intervention group
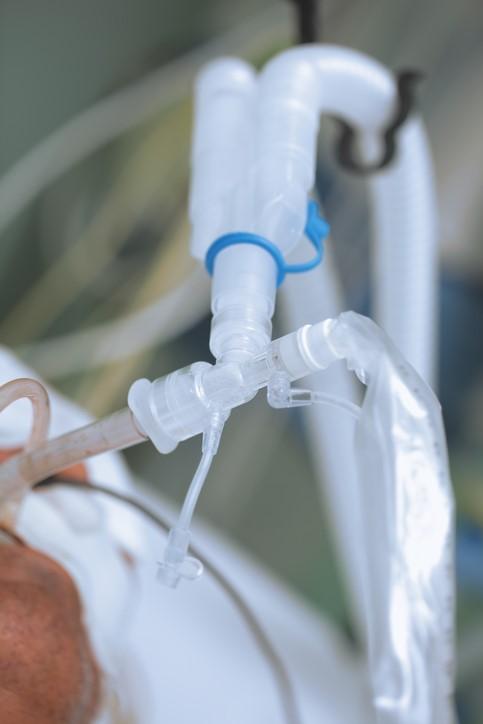
In the VAPrapid2 trial, conducted from November 2013 to September 2016, 210 adult ICU patients with suspected VAP were randomly assigned to receive biomarker-guided recommendation on antibiotics (the intervention group) or routine use of antibiotics (the control group). For the 104 patients in the intervention group, antibiotic recommendations were based on the levels of IL-1β and IL-8 in bronchoalveolar lavage fluid, which is squirted through a bronchoscope into a small part of the lung and then collected for analysis.
Bronchoalveolar lavage was performed on all patients in the trial. But if the concentrations of IL-1β and IL-8 were below a previously validated cutoff in the intervention group patients, clinicians were notified that VAP was unlikely and advised that they should consider discontinuing antibiotics. While clinicians in the trial were encouraged to follow the biomarker-guided recommendations, antibiotic use was ultimately at their discretion.
The primary outcome of the study was the distribution of antibiotic-free days in the 7 days following bronchoalveolar lavage.
The results of the trial showed no difference in antibiotic-free days between the two groups, with patients in both groups receiving a median of 6 days of antibiotics in the 7 days following the procedure. In the biomarker-guided group, the IL-1β and IL-8 level was high in 64 patients and low in 17 patients; the recommendation to discontinue antibiotics was followed in only 4 of those patients, with 1 patient having a false-negative result. Twenty-two of the assay tests failed.
Overall, 70 of the 209 participants (1 was withdrawn) had microbiologically confirmed VAP—38 in the in the intervention group and 32 in the control group. A total of 170 received antibiotics.
'Deeply entrenched' prescribing habits
A process evaluation conducted by the trial investigators provides some clues as to why the test failed to curtail antibiotic use.
Analysis of data and interviews with investigators found that clinician skepticism about the additional diagnostic value of bronchoalveolar lavage, which is not considered a gold-standard diagnostic and remains contentious, may have affected recruitment and compliance with the study. Low recruitment in some of the participating ICUs appeared to correspond with less use of the test outside of the trial, along with a culture of not actively de-escalating antibiotics and an absence of trial champions.
Moreover, the evaluation found that "deeply entrenched prescribing characteristics" and concern about the risk of failing to treat potential VAP cases likely influenced the decision to continue antibiotics in many of the patients who had low IL-1β and IL-8 levels.
"Our data suggest that the observed absence of effect was more likely explained by clinicians' behaviour than by poor test performance," the authors of the study write. "Beliefs around assumed efficacy and safety of antibiotics shape prescribing in emergency departments and presumably the motivation to avoid harm is enhanced in suspected ventilator-associated pneumonia in the ICU."
In an accompanying commentary, infectious disease physician Sarah Doernberg, MD, of the University of California, San Francisco, suggests that unless some of these deeply held beliefs about antibiotic use are addressed, even the most precise diagnostic test won't help improve antibiotic stewardship.
"In the age of precision medicine, as more diagnostic tests to identify pathogens, the microbiome, and host response come to market, it is crucial that the study of implementation science and a deep understanding of what motivates antibiotic prescribing advance simultaneously," Doernberg writes. "We might not need better biomarkers, but instead need more effective strategies to implement the knowledge we have into practice. Without these strategies we will be left with another test that will add cost but not value to the care we provide to patients."
See also:
Dec 3 Lancet Respir Med study
Dec 3 Lancet Respir Med commentary