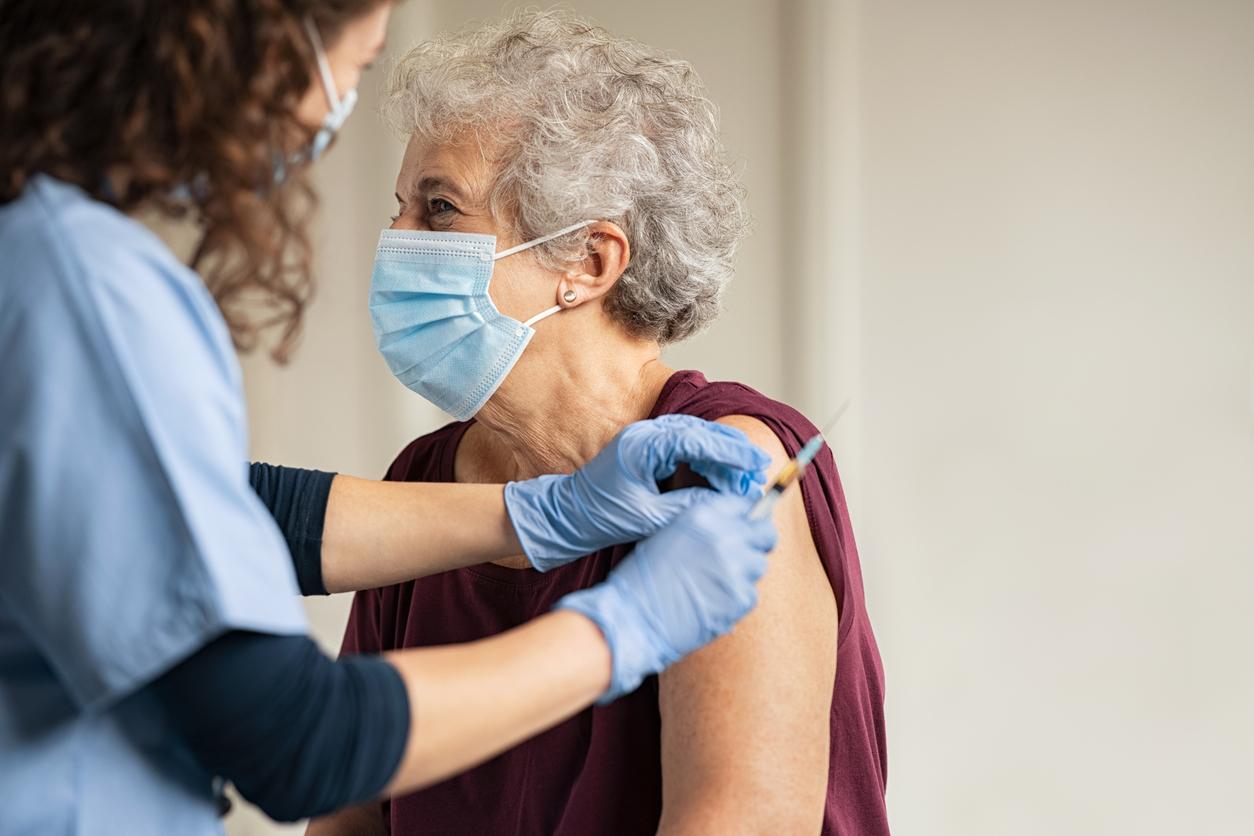
In an emergency session today, the vaccine advisory panel for the Centers for Disease Control and Prevention (CDC) voted to recommend that Americans receive the Pfizer-BioNTech COVID-19 vaccine, a key step that allows healthcare workers to begin administering the vaccine to people.
The interim recommendation covers people ages 16 years and older under the Food and Drug Administration (FDA) emergency use authorization (EUA).
The decision comes a day after the FDA issued its EUA, a step that cleared the way for the vaccine to begin shipping.
The vote from the CDC's Advisory Committee on Immunization Practices (ACIP) passed with 11 votes in favor and 3 recusals. A separate vote to amend the adult and child immunization schedule passed unanimously by the full voting group.
During the public comment session ahead of the vote, some groups spoke out against the vaccine, but most supported it and wanted specific groups included in future prioritization decisions, such as people in incarceration settings, those in senior living centers, teachers, and food workers. Public health groups also asked for more resources to help administer the vaccine.
Earlier during today's deliberations, CDC officials clarified some issued related to the vaccine, including that the Pfizer-BioNTech vaccine isn't interchangeable with other COVID-19 vaccines.
They also said the vaccine should be offered to people who were previously infected, though people with current infections should wait until they have recovered. Officials also said those with known exposure should wait until their quarantine period expires to avoid potentially exposing healthcare workers and others at immunization sites.
ACIP reviews vaccine data before recommending any vaccine and issues guidance for their use.