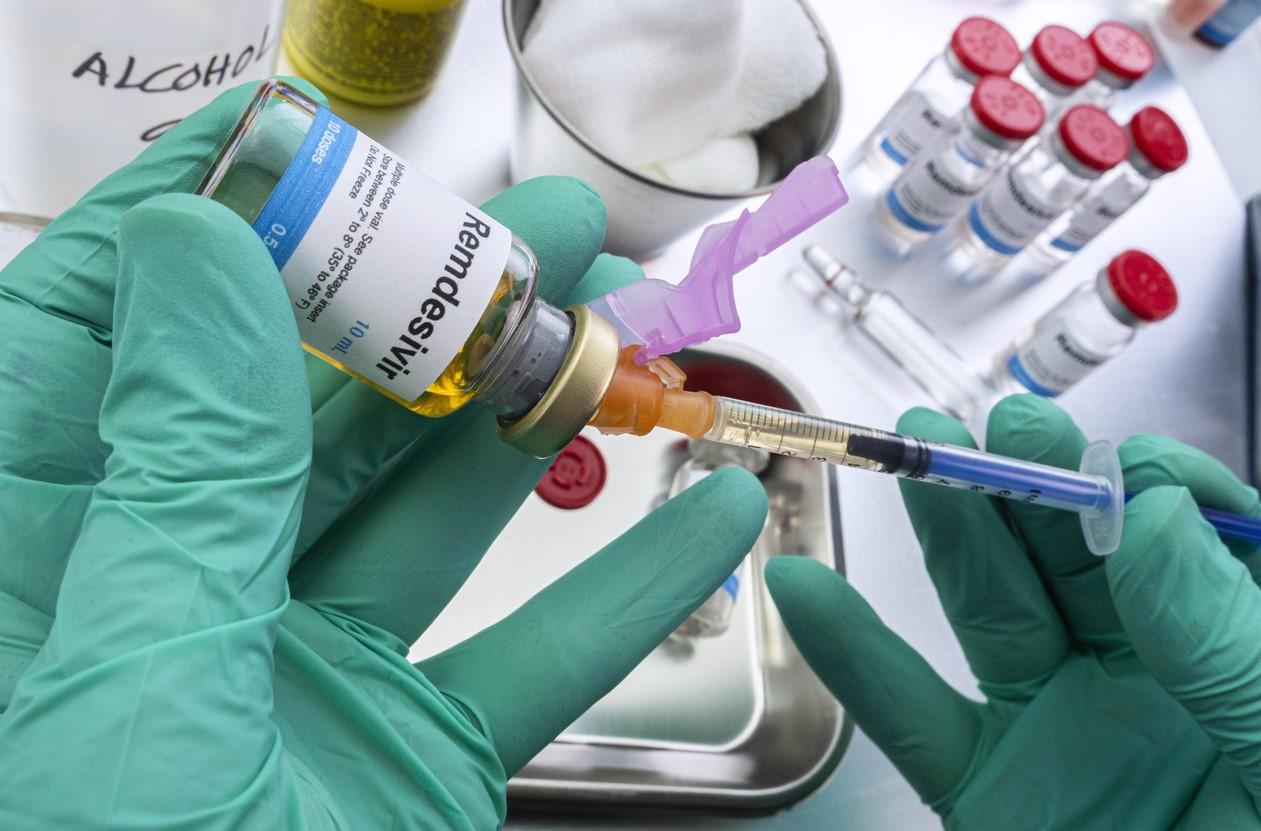
A comparative-effectiveness study that spanned the dominance of SARS-CoV-2 variants of concern (VOC) finds that the antiviral drug remdesivir (Veklury) significantly reduced in-hospital COVID-19 death rates among adults receiving supplemental oxygen on admission.
The study, led by researchers from remdesivir manufacturer Gilead Sciences, was published today in Open Forum Infectious Diseases.
The team compared 67,582 hospitalized COVID-19 patients receiving low-flow oxygen (LFO), 34,857 on high-flow oxygen/noninvasive ventilation (HFO/NIV), and 4,164 receiving invasive mechanical ventilation/extracorporeal membrane oxygen (IMV/ECMO) treated with remdesivir with control patients from December 2020 to April 2022. At least one dose of remdesivir was administered within 2 days of hospitalization.
The study authors noted that World Health Organization guidelines conditionally recommend remdesivir for severely—but not critically—ill COVID-19 patients. "This lack of recommendation for remdesivir use in critically ill patients may reflect either lower efficacy or alternatively, the inability to detect beneficial effects, since clinical trials were not designed or powered to detect differences in remdesivir efficacy in subgroups according to baseline COVID-19 severity," they wrote.
Drug appears protective across variants
Across VOC periods, 5,853 (16.8%) remdesivir recipients and 6,770 (19.4%) controls died by 14 days. By 28 days, 9,009 (25.8%) remdesivir recipients and 9,853 (28.3%) controls died.
In the US, approximately 1 in 2 patients requiring invasive ventilation has died since the beginning of the pandemic.
Unadjusted death rates were significantly lower for remdesivir recipients than controls at 14 days (LFO, 6.4% vs 8.8%; HFO/NIV, 16.8% vs 19.4%; IMV/ECMO, 27.8% vs 35.3%) and 28 days (LFO, 9.8% vs 12.3%; HFO/NIV, 25.8% vs 28.3%; IMV/ECMO, 41.4% vs 50.6%).
After adjustment, remdesivir was tied to significantly lower in-hospital death rates at 14 days (LFO adjusted hazard ratio [aHR], 0.72; HFO/NIV aHR, 0.83; IMV/ECMO aHR, 0.73) and 28 days (LFO aHR, 0.79; HFO/NIV aHR, 0.88; IMV/ECMO aHR, 0.74) relative to no remdesivir.
"In the US, approximately 1 in 2 patients requiring invasive ventilation has died since the beginning of the pandemic," wrote the authors, who said remdesivir should be administered immediately to all hospitalized COVID-19 patients. "It remains essential to continue to evaluate therapeutic options to treat patients throughout the spectrum of COVID-19 disease and VOC periods."