An mpox outbreak in the Democratic Republic of the Congo (DRC) continues to spread within the country, with the first case reported in North Kivu province, according to a recent update from the World Health Organization (WHO).
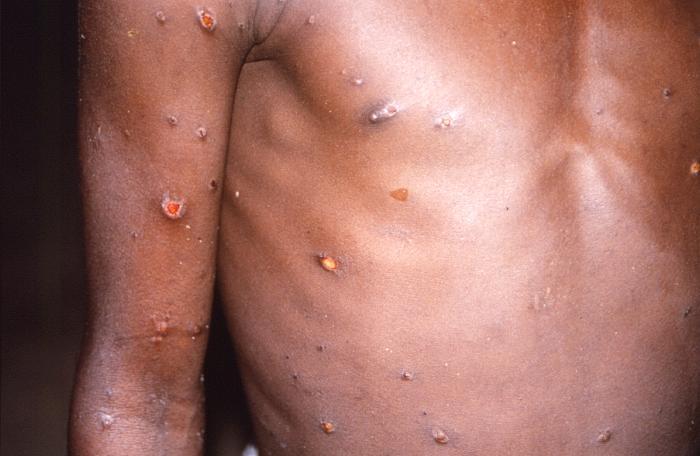
The DRC's outbreak began in late 2022 and involves the clade 1 mpox virus, which is different from the clade 2 strain circulating globally. The outbreak is notable, because it is the first in Africa—where much of the disease activity has been related to zoonotic spread—to involve sexual spread. One of the country's hot spots has been Kamatuga in South Kivu province, where a novel clade 1 virus emerged with mutations that make the virus more adapted to circulation among humans. Sequencing suggests, however, that viruses from other parts of the country don't have the mutations.
The case in North Kivu province involves a 19-year-old woman whose illness was confirmed in Goma on June 1. Investigators found that she had sexual contact with a person with a suspected infection who had been in South Kivu province.
So far in 2024, 7,851 mpox cases have been reported in the DRC, 384 of them fatal. With the case from Noth Kivu province, illnesses have now been reported in 23 of the country's 26 provinces. The disease is spreading through various contact forms, including sexual, nonsexual, household, and healthcare.
Children are still the most affected age-group, with 39% of cases in kids younger than 5 years old. The disease was fatal in 240 of those cases.
Outbreak in South Africa, case rise in Los Angeles
South Africa recently reported an mpox outbreak involving the clade 2 virus responsible for global spread. So far, seven cases have been reported, according an update from the Africa Centres for Disease Control and Prevention. The country also reported its first two mpox deaths. All cases were severe and involved men ages 30 to 39 who had underlying health conditions such as HIV. So far, investigations have found the patients hadn't traveled to countries that have ongoing outbreaks.
In the United States, the Los Angeles Department of Public Health yesterday posted an alert about a rise in mpox cases, with 10 illnesses reported in the past 2 weeks. For comparison, it said during the previous several weeks it was averaging about 2 cases a week. It urged people in risk groups to take precautions and to get vaccinated.