Sep 10, 2004 (CIDRAP News) A test of smallpox vaccine made by Aventis Pasteur in the 1950s show it is still effective even when diluted, suggesting that the United States has more than enough vaccine for everyone, according to a report this week in the Journal of the American Medical Association.
A trial of the vaccine in 340 young adults showed it was effective when diluted to one-fifth and one-tenth of full strength, according to the report by a team of 10 researchers from Vanderbilt University, the University of Iowa, Cincinnati Children's Hospital, and EMMES Corp. The lead author is Thomas R. Talbot, MD, MPH, of Vanderbilt University School of Medicine.
The existence of Aventis Pasteur's stockpile of millions of doses of frozen smallpox vaccine was publicly disclosed in March 2002. The company then offered to turn the vaccine over to the federal government, which was preparing a smallpox vaccination program for the military and some healthcare workers at the time. The JAMA report says the stockpile contains about 85 million doses.
Before the disclosure of the Aventis stockpile, the nation's supply of smallpox vaccine was only about 15 million doses (though studies have shown it can be diluted). However, the British vaccine maker Acambis has been under contract since 2001 to supply 209 million doses of a smallpox vaccine to the government.
Using vaccine made in 1956 and 1957, the recent study was a double-blind, randomized trial funded by the National Institute of Allergy and Infectious Diseases. Volunteers recruited at Vanderbilt, Iowa, and Cincinnati were screened for contraindications and given the vaccine at full strength or in a 1:5 or 1:10 dilution. The volunteers, who had never had a smallpox shot, were then observed in person every 3 to 5 days for 2 weeks and again at 1 and 2 months after vaccination, and were interviewed by phone after 6 months. Blood serum samples were taken before vaccination and 28 and 56 days afterward.
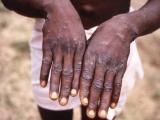
More than 99% of the vaccinees showed a vaccination "take," defined as a vesicle or pustule at the site 6 to 11 days after vaccination, with no significant difference in take rates between the three dilution groups, the report says. All volunteers had induration and erythema at the inoculation site, but the areas affected were significantly smaller in women than in men.
Because of the number of smallpox vaccine trials coinciding with this one, serologic data for all the vaccinees were not available in time for the JAMA report. However, data were available for a subset of 109 volunteers from Vanderbilt University. By a month after vaccination, all except one of these vaccinees had developed a neutralizing antibody response. This response was significantly higher in the 1:5 dilution group than in the 1:10 group.
In the 2 weeks after vaccination, 99.7% of the volunteers had pain or itching at or near the vaccination site. Systemic symptoms were also common: fatigue was reported by 79.1%, myalgia by 78.2%, headache by 75.0%, and fever by 21.5%. The frequency of these side effects was about the same in all three groups. A quarter of the vaccinees missed some activities because of reactions to the shot. The authors say the frequency of side effects, while not unexpected, seemed higher than that in a recent trial of lyophilized (powdered) smallpox vaccine.
The researchers did not actively monitor the volunteers for cardiac symptoms, because the trial took place before myopericarditis emerged as a rare side effect in the US military smallpox vaccination program. However, four volunteers reported episodes of chest pain or tightness in the 2 weeks after vaccination, and one reported exercise-associated dyspnea and tachycardia. All of these symptoms resolved with rest or treatment with nonprescription drugs, the report says.
In view of the findings, the authors write, "The existing supply of approximately 85 million doses of APSV [Aventis Pasteur smallpox vaccine] can be expanded, leaving an ample stockpile of smallpox vaccine to protect the entire US population in the event widespread vaccination is imminently needed. With adequate supplies of vaccine for the population of the United States, the potential exists for sharing additional supplies with other countries as well."
Talbot TR, Stapleton JT, Brady R, et al. Vaccination success rate and reaction profile with diluted and undiluted smallpox vaccine. JAMA 2004;292(10):1205-12 [Abstract]