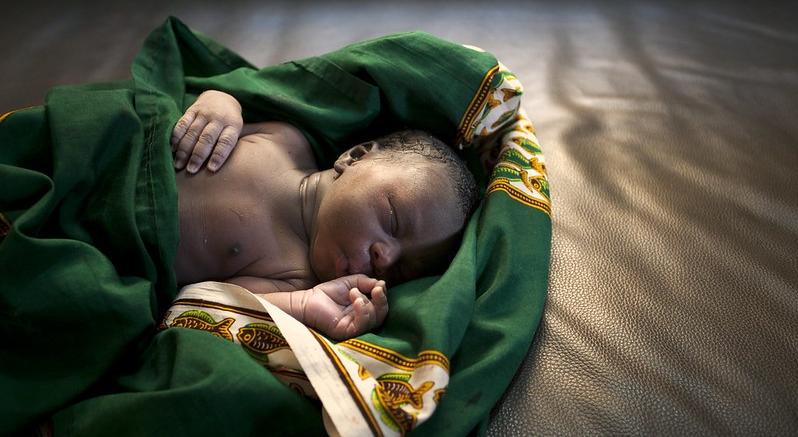
A randomized clinical trial in West Africa found that receipt of the antibiotic azithromycin during labor did not reduce the incidence of neonatal sepsis or mortality, researchers reported today in JAMA.
The trial, which involved nearly 12,000 pregnant women in The Gambia and Burkina Faso, found that the incidence of neonatal mortality or sepsis was similar whether mothers received an oral dose of azithromycin or placebo, though the intervention did significantly reduce other, noninvasive infections in newborns and their mothers.
Sepsis, which occurs when the immune system has an overwhelming reaction to a bacterial or viral infection, accounts for nearly one third of newborn deaths globally. Its impact is acutely felt in sub-Saharan Africa, which has some of the highest neonatal mortality rates worldwide.
Trial investigators had hoped that azithromycin might decrease neonatal sepsis by reducing carriage of bacterial pathogens that can cause invasive infections in newborns. But that hypothesis was not borne out by the results.
"These results do not support the routine introduction of oral intrapartum azithromycin for this purpose," they wrote.
No difference in primary end point
For the PregnAnzi-2 (Pre-delivery Administration of Azithromycin to Prevent Neonatal Sepsis & Deaths) trial, investigators with the Medical Research Unit The Gambia at the London School of Hygiene and Tropical Medicine and the Institut de Recherche en Sciences de la Santé–Clinical in Burkina Faso recruited pregnant women from 10 health facilities in the two countries from October 2017 through October 2021.
Both countries have similar rates of neonatal mortality, with 26 and 30 deaths per 1,000 live births in The Gambia and Burkina Faso, respectively.
Participants were randomly assigned to receive either 2 grams of oral azithromycin during labor or placebo. The primary end point was a composite of sepsis or death during the first 28 days. Secondary end points included skin infections in newborns and sepsis, mastitis, and puerperal fever in birth mothers.
Of the 14,554 women assessed for eligibility, 11,983 were recruited and randomized, with 11,625 parents and 11,783 newborns included in the 28-day analysis. In the primary analysis, 225 newborns (1.9%) met the primary end point (133 with sepsis, 68 deaths, and 24 with both). The incidence of neonatal sepsis or mortality was 2.0% in the azithromycin group and 1.9% in the placebo group (risk difference [RD], 0.09; 95% confidence interval [CI], –0.39 to 0.57; odds ratio, 1.06; 95% CI, 0.80 to 1.38).
These results do not support the routine introduction of oral intrapartum azithromycin for this purpose.
Mortality was slightly lower in the azithromycin group than the placebo group in The Gambia (0.6% vs 1.0%) but higher in the azithromycin group in Burkina Faso (1.0% vs 0.5%). The opposite trend was observed for sepsis.
Gram-negative bacteria accounted for 22 of 37 (59.5%) confirmed sepsis cases (69.2% in the azithromycin group vs 54.2% in the placebo group), and Staphylococcus aureus was the most commonly isolated pathogen (21.6%).
Analysis of secondary outcomes showed that clinical skin infections in newborns were reduced by more than half (0.8% vs 1.7%; RD, –0.90; 95% CI, –1.30 to –0.49) and later need for antibiotics was lower (6.2% vs 7.8%; RD, –1.58; 95% CI, –2.49 to –0.67) in the azithromycin group. Postpartum women who received azithromycin had a lower incidence of mastitis (0.3% vs 0.5%; RD, –0.24; 95% CI, –0.47 to –0.01) and puerperal fever (0.1% vs 0.3%; RD, –0.19; 95% CI, –0.36 to –0.01).
'Azithromycin is not likely a solution'
The PregnAnzi-2 trial is the latest to investigate the potential benefits of the widely used macrolide antibiotic in reducing childhood mortality in sub-Saharan Africa. Among them is the MORDOR trial, which found that twice-a-year administration of azithromycin in rural communities in Tanzania, Malawi, and Niger was associated with a 14% reduction in mortality in children under 5, with the strongest effect seen in children younger than 6 months. Even though the exact mechanism is unclear, the trial has sparked interest, and similar studies, in other African countries.
In 2016, several of the PregnAnzi-2 investigators conducted a proof-of-concept study that found oral azithromycin administered during labor reduced carriage of gram-positive bacteria in mothers and newborns during the subsequent 4 weeks—a result that prompted the current trial. The hope was that reducing carriage of certain bacterial pathogens might lower neonatal sepsis and mortality by limiting the transmission of those pathogens from mother to newborn during childbirth.
But the results from PregnAnzi-2 are consistent with another trial conducted in seven countries (three in Africa) that found that a dose of azithromycin before vaginal delivery, while cutting risk of maternal sepsis by 35%, did not reduce the incidence of stillbirths or neonatal sepsis.
Antibiotics can cause serious adverse events in pregnant women, promote antibiotic resistance, and disrupt the still-developing microbiome of infants.
In an accompanying editorial, researchers with the US Centers for Disease Control and Prevention and Emory University say there are reasons to be skeptical of using azithromycin to reduce neonatal sepsis. They note in particular that azithromycin isn't the preferred antibiotic for group B Streptococcus or Escherichia coli, which are two of the leading causes of early-onset neonatal sepsis.
They also point out that antibiotics can cause serious adverse events in pregnant women, promote antibiotic resistance, and disrupt the still-developing microbiome of infants, which has been associated with long-range health impacts.
"In this light, even though the trial did report reductions in some secondary end points, including skin infections in newborns and mastitis and puerperal fever in birthing parents, the failure of the intervention to prevent the primary outcome provides compelling information that intrapartum azithromycin is not likely a solution for preventing neonatal sepsis and its associated mortality," they wrote.