- Part of an ongoing surge in dengue activity, the Brazil's health ministry said suspected cases this week topped 1 million, according to the country's weekly arbovirus surveillance report. The Americas region reported record dengue activity in 2023, and the Pan American Health Organization recently warned that activity so far this year is already outpacing last year. Peru this week declared a health emergency for dengue for most of its regions.
- Five states have reported more highly pathogenic avian flu outbreaks in poultry over the past few days, including Missouri, where the virus struck a commercial turkey farm in Dallas County, according to updated data from the US Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS). Four other states reported outbreaks in backyard flocks, including Indiana, Minnesota, New York, and Ohio.
- One country—Nigeria—reported a new polio case this week, according to the latest weekly update from the Global Polio Eradication Initiative. The case involved circulating vaccine-derive poliovirus type 2. The patient is from Kano state, and his or her paralysis onset occurred in November 2023, boosting the country's total for 2023 to 82.
Quick takes: A million dengue cases, more avian flu in 5 states, polio in Nigeria
Research: Behavior change had larger role than vaccination in curbing 2022 mpox epidemic
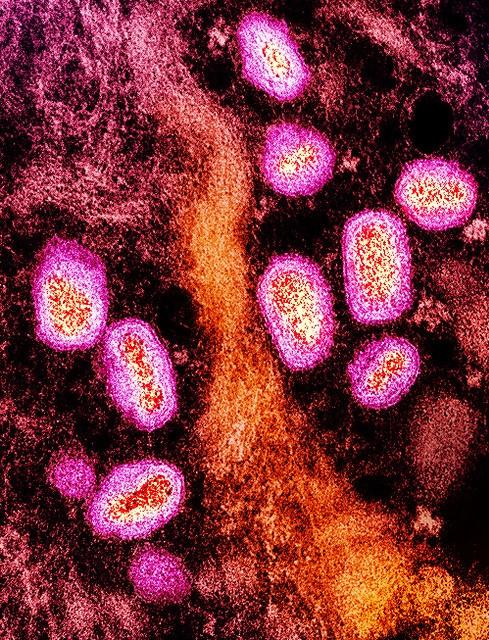
A University of Washington analysis reveals that mpox spread in North America started to slow down before 10% of high-risk people in the United States were vaccinated, suggesting that behavior change helped end the 2022 outbreaks.
For the study, published yesterday in Cell, investigators built models to analyze global and regional transmission dynamics using mpox virus genomes from five global regions, as well as air traffic and epidemiologic data.
The multicountry mpox outbreaks, which occurred mainly among men who have sex with men (MSM), were unusual in that they occurred in non-endemic regions. In July 2022, the World Health Organization declared the primarily sexually transmitted infection a public health emergency of international concern.
Surveillance, behavior change may have been sufficient
In the 2022 epidemic, mpox was first detected in the United Kingdom on May 7, followed by rapid transmission around the world. Most cases were initially reported in Western and Southern, and then Central Europe, peaking in mid-July. Genetic sequences traced the most common viral ancestor of the epidemic to Western Europe from March 9 to 27.
In North America, cases were first reported in mid-May, ultimately leading to the largest outbreak in the world and peaking in early August. Cases were first detected in South America in mid-May, making up a large proportion of cases at the end of the epidemic.
These findings highlight the significant effect of behavioral change among MSM in curbing the epidemic as well as emphasize the need for prompt public health response in order to maximize the population-level effectiveness of vaccination campaigns.
Genetic sequences from submitted samples showed diversity among regions and in North America. Disease transmission in North America started to drop before more than 10% of high-risk Americans were vaccinated. The authors found strong evidence of viral circulation before initial case detection in each affected world region.
"These findings highlight the significant effect of behavioral change among MSM in curbing the epidemic as well as emphasize the need for prompt public health response in order to maximize the population-level effectiveness of vaccination campaigns," the researchers wrote.
The results, they said, underscore the importance of routine specimen screening for emerging infectious diseases and of mining genomic and epidemiologic data for outbreak mitigation.
WHO shares details of 2 fatal Nipah cases in Bangladesh
The World Health Organization (WHO) this week fleshed out details on Nipah virus infections in Bangladesh that first surfaced in media reports in late January.
The two cases were confirmed before February 9, and both patients died from their infections. Both are from Dhaka division but are from separate districts and aren't epidemiologically linked to each other, the WHO said in a statement.
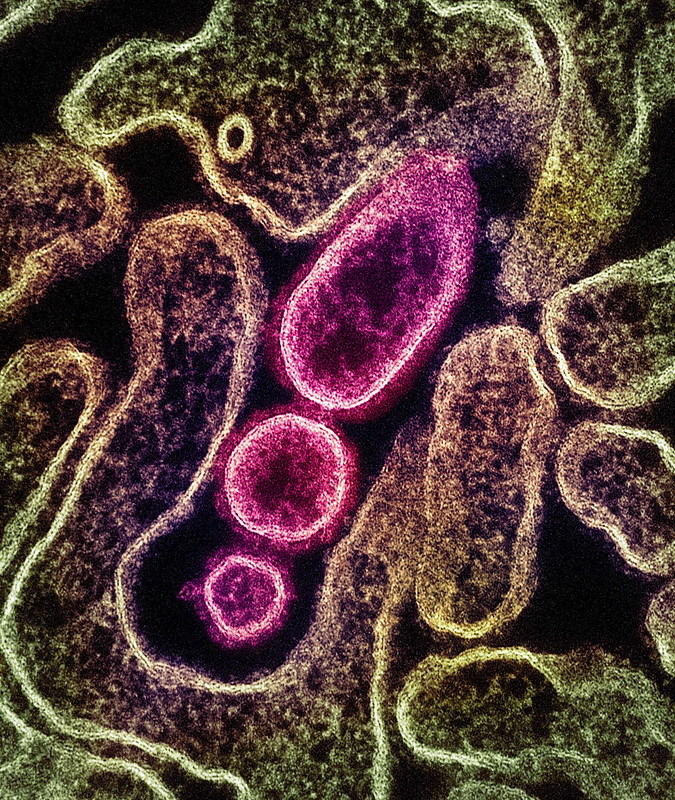
Man had consumed raw date palm sap
The first patient is a 38-year-old man whose symptoms began on January 11. He was admitted to a local hospital on January 16, then transferred to a facility in Dhaka city when his condition worsened. The man died on January 28 about a week after his blood and respiratory samples tested positive for Nipah virus.
An investigation revealed the man had consumed raw date palm sap on December 31, a practice known to increase the risk of contracting the disease. No other Nipah cases were detected in 91 of the man's contacts. Infections in Bangladesh typically track with harvest of date palm sap, which occurs from December through May. Public health officials continue to warn against drinking raw date palm sap because it can be contaminated with bat droppings that contain the virus.
Girl regularly drank raw date palm sap
The second patient is a 3-year-old girl who was isolated in a Dhaka city hospital on January 30, about 2 days after her symptoms began. On the following day her Nipah virus infection was confirmed and she died.
Investigators found that the girl had regularly consumed raw date palm sap. All 67 of the girl's contacts tested negative for the virus.
The WHO said the risk to Bangladesh is moderate, partly due to the high case-fatality rate and because people continue to drink raw date palm sap, despite ongoing efforts to teach communities about the risk. It added that the regional risk is moderate because Bangladesh shares an ecological corridor with India and Myanmar for the bats that are the natural Nipah virus hosts.
There are no vaccines or treatments for Nipah virus, which has been classified as a priority disease for countermeasure research and development.
AMR Industry Alliance calls for 'bold, coordinated' action on antimicrobial resistance

The AMR Industry Alliance this week issued a "call-to-action" in advance of the United Nations (UN) High-Level Meeting on AMR (antimicrobial resistance) in September.
The document from the Alliance, which represents companies and trade associations from the pharmaceutical, biotechnology, generics, and diagnostics industries, calls on the UN and its member states to take "bold, coordinated" action against AMR in four key areas: antibiotic manufacturing, antibiotic research and development, access, and appropriate use.
To strengthen antibiotic manufacturing standards and reduce the amount of antibiotic pollution released into the environment, the Alliance urges the UN and member states to adopt the Antibiotic Manufacturing Standard published by the group in 2022 as part of their tendering and reimbursement policies. It also calls for incentivizing all antibiotic manufactures and suppliers to seek the Minimized Risk of Antimicrobial Resistance certification developed by the Alliance with the British Standards Institution.
To address antibiotic market failures and ensure a functioning market for new antibiotics, the document calls for governments to develop new financial incentives that encourage companies and investors to pursue antibiotic innovation.
Combatting AMR requires a broad, multi-stakeholder approach.
To boost access to antibiotics, particularly in lower-resources countries, the Alliance urges the removal of barriers to registration, acceleration of the approval process for new antibiotics, and reform of antibiotic procurement policies. And to ensure that new antibiotics are being used appropriately, the Alliance calls for the UN and its member states to implement stewardship programs, use diagnostic tests, prioritize investments in laboratory infrastructure, and expand AMR surveillance at local, national, and global levels.
'Critical opportunity' for countries to address AMR
Alliance Board Chair James Anderson says the UN High-Level Meeting on AMR—the second since 2016—is a "critical opportunity" for the private sector to work with governments, international organizations, civil society, and other stakeholder to address the AMR crisis.
"Combatting AMR requires a broad, multi-stakeholder approach," Anderson said in a news release. "We're pleased to launch industry's call-to-action to drive cooperation with our private and public sector partners as we approach this pivotal moment."
CARB-X funds development of novel vaccine for infant sepsis
CARB-X announced yesterday that it is awarding Danish research and development company GlyProVac $467,000 to develop a maternal vaccine that targets a leading cause of neonatal sepsis.
The award from CARB-X (Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator) will support the development GPV02, a novel vaccine that uses a selected bacterial protein naturally decorated with small sugar molecules to trigger an immune response against Escherichia coli. Vaccinated mothers would pass the antibodies on to their babies in utero and through breastmilk to help their newborns' ward off an E coli infection. The vaccine also has the potential to prevent urinary tract infections caused by E coli.
An estimated 2.5 million newborns or infants in the first month of life, primarily in low-and middle-income countries, die each year from sepsis, which occurs when a bloodstream infection triggers a life-threatening immune response. E coli is one of the primary causes.
4th vaccine to receive CARB-X funds
"E. coli can cause a range of infections in humans, including urinary tract infections and neonatal sepsis in newborns, and poses a major challenge for public healthcare systems," Anders Boysen, PhD, CEO and Co-founder of GlyProVac, said in a CARB-X press release. "We are grateful for the invaluable support, network, and resources provided by CARB-X, which will give us the opportunity to expand the potential of our E. coli vaccine to accelerate the fight against neonatal sepsis."
E. coli can cause a range of infections in humans.
GPV02 is the seventh project, and fourth vaccine, to receive funding from CARB-X's 2022-2023 funding call. Earlier this year, CARB-X awarded Syntiron, of St. Paul, Minnesota, $1.7 million to develop its Alloy-EK vaccine, which targets E coli and Klebsiella pneumoniae, for neonatal sepsis.
In case you missed it
This week's top reads
Our underwriters
Unrestricted financial support provided by












