The results of a randomized clinical trial conducted in Japan indicate that Gram stain–guided antibiotic therapy could help safely reduce the use of broad-spectrum antibiotics in patients who have ventilator-associated pneumonia (VAP).
The results, published last week in JAMA Network Open, showed that Gram stain–guided antibiotic treatment in VAP patients yielded non-inferior clinical responses to guideline-based treatment, while significantly reducing the use of antipseudomonal and anti-methicillin resistant Staphylococcus aureus (MRSA) antibiotics.
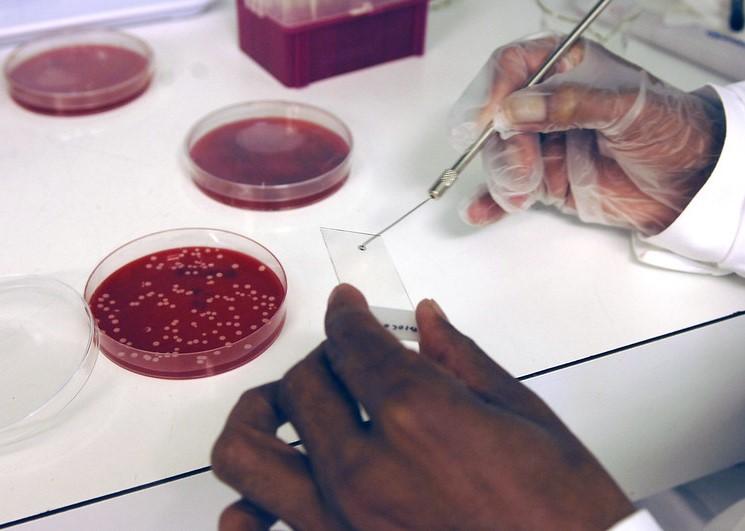
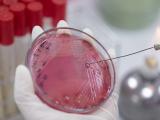
A Gram stain is an inexpensive, simple test—invented by Danish bacteriologist Hans Christian Gram in 1884—that uses samples from the site of an infection to determine whether the infection is caused by gram-positive bacteria (signified by a purple stain) or gram-negative (a red or pink stain). The test is used worldwide to guide initial antibiotic therapy for a variety of bacterial infections, but questions have remained about whether it is accurate enough to safely restrict broad-spectrum antibiotic use.
The investigators wanted to test this technique for suspected pneumonia patients, including VAP, because of concerns that current treatment guidelines result in overuse of broad-spectrum agents, which can promote antibiotic resistance.
They say the results suggest that use of the "time-honored" Gram stain technique may be a better option than more expensive molecular point-of-care tests for optimizing antibiotic choice in pneumonia and slowing the spread of multidrug-resistant (MDR) bacteria.
Similar clinical results
For the GRACE-VAP (Gram Stain–Guided Antibiotic Choice for VAP) trial, a team of investigators recruited VAP patients from intensive care units (ICUs) at 12 Japanese hospitals from April 2018 through May 2020. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
The patients were randomly assigned 1:1 to Gram stain–guided antibiotic therapy (using endotracheal aspirates) or guideline-based antibiotic therapy based on the 2016 Infectious Diseases Society of America and American Thoracic Society VAP clinical practical guidelines for VAP. Both of those guidelines recommend empirical coverage for both MRSA and Pseudomonas aeruginosa.
The primary outcome was the rate of clinical response, defined as completion of antibiotic therapy within 14 days, improvement or lack of progression of baseline radiographic findings, resolution and signs and symptoms of pneumonia, and lack of antibiotic agent readministration. The non-inferiority margin was 20%.
Secondary outcomes included the proportions of anti-MRSA or antipseudomonal agents used as initial therapy, 28-day mortality, intensive care unit (ICU)-free days, ventilator-free days, and adverse events.
A total of 206 patients (median age 69 years, 68.4% men) were included in the per-protocol analysis, with 103 in each group. The most frequently isolated bacteria from endotracheal aspirate were S aureus (50%), followed by Klebsiella spp (16.5%) and Haemophilus influenzae (9.7%).
Clinical response occurred in 79 patients (76.7%) in the Gram stain–guided group and 74 patients (71.8%) in the guideline-based group, for a risk difference of 0.05 (95% confidence interval [CI], 0.07 to 0.17; P < .001). The 28-day cumulative incidence of mortality was 13.6% in the Gram stain–guided group versus 17.5% in the guideline-based group. There were no significant differences between the groups for ICU-free days, ventilator-free days, or adverse events.
Compared with the guideline-based treatment group, the use of anti-MRSA antibiotics was reduced by 38.8% (95% CI, 29.4% to 48.9%) in the Gram stain–guided group, and the use of antipseudomonal agents was reduced by 30.1% (95% CI, 21.5% to 39.9%). Escalation of antibiotics according to culture results was performed in 7 patients (6.8%) in the Gram stain–guided group, compared with 1 patient (1%) in the guideline-based group.
"Gram stain–guided restrictive antibiotic therapy was noninferior to guideline-based broad-spectrum antibiotic therapy in patients with VAP in terms of the clinical response rate," the authors wrote. "Gram staining of endotracheal aspirates optimized the use of broad-spectrum antibiotic agents for VAP without detrimental effects on patient outcomes."
They added, "This finding reinforces the potential use of Gram staining in the critical care setting to ameliorate the spread of MDR pathogens."